How can we capture the therapeutic potential that psychedelic drugs have demonstrated against mental disorders while fading their risks in the discovery of new medicines?
A key insight highlighted using fluorescent biosensors is that non-hallucinogenic analogues of these drugs are characterised by relatively modest intrinsic efficacy at the serotonin 5-HT2A receptor.
Psychedelic drugs that activate the serotonin 5-HT2A receptor (5-HT2AR) have gained massive scientific interest for their therapeutic potential against a range of psychiatric disorders, including depression, anxiety, obsessive-compulsive disorder, and addiction1. In particular, many researchers are considering approaches for developing non-hallucinogenic analogues to make these treatments more scalable, affordable, and applicable to larger populations due to the extensive medical supervision required in the current model of psychedelic-assisted psychotherapy and the counter-indications against patients with risks of psychosis and self-harm2-4. However, greater evidence is necessary on the molecular mechanisms by which psychedelics produce their hallucinogenic effects through the 5-HT2AR, with different studies indicating that either strong intrinsic efficacy5 or biased agonism between G protein signalling and β-arrestin recruitment pathways could be responsible6-9. Notably, this investigation is challenged by various methodological factors such as the sensitivity, time-dependence, and physiological relevance of bioassays that must be addressed to effectively determine and translate drug activity.
In a recent study published by British Journal of Pharmacology, University of Oxford researchers in collaboration with the biotechnology company Compass Pathways implemented Montana Molecular’s fluorescent biosensors to elucidate the mechanism of psychedelic drug actions at the 5-HT2AR10. Towards addressing this need, the authors examined various 5-HT2AR agonists at the Gαq/11 signalling and β-arrestin recruitment pathways in recombinant human SH-SY5Y neuroblastoma cells (Figure 1). Using Montana Molecular’s Borealis biosensor and the associated signalling kinetics analyses developed by Dr Sam Hoare11-13 enabled the authors to reliably and accurately measure β-arrestin recruitment to the 5-HT2AR with several practical advantages. The assay operated with an unmodified version of the receptor, important for preserving its native conformational dynamics and functionality14. The G protein-coupled receptor (GPCR) kinases (GRKs) included in the kit were crucial for potentiating the interaction between the 5-HT2AR and β-arrestin to ensure that a response was detected even from weak agonists, avoiding a misleading lack of response that is commonly found in other assays of this pathway, due to the fact that it contains no inherent signal amplification15-18. Being able to monitor β-arrestin recruitment in real-time with the fluorescent biosensor facilitated quantification of the steady state response, avoiding inconsistent comparisons of activity that can arise at early timepoints when the response is unknowingly incomplete11,13,15,18-23. The BacMam vector packaging the 5-HT2AR, β-arrestin recruitment biosensor, and GPCR kinase allowed these components of the assay to be abundantly expressed with a simple workflow and minimal variability in cells of biological relevance to the human brain. SH-SY5Y cells are known to more appropriately represent neuronal function than commonly used human embryonic kidney 293 (HEK293) or Chinese hamster ovary (CHO) cells and were compatibly employed for this reason despite their typically lower transfectability24-28. Lastly, the readily titratable nature of this expression provided the capability to adjust the sensitivity of the assay to ensure that all drugs produced a partial agonist maximal response, which is essential for interpreting differences in intrinsic efficacy16,29.
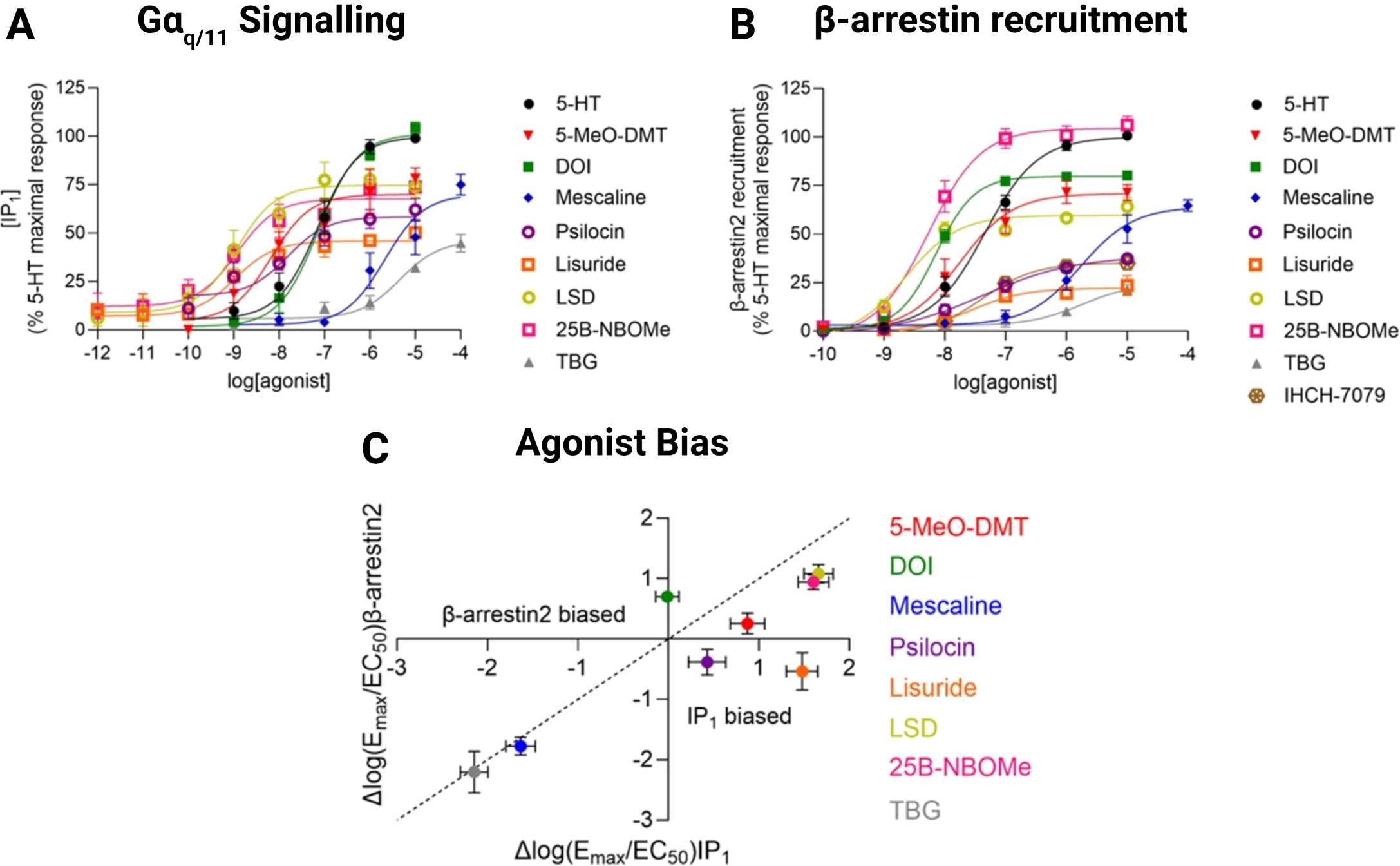
Figure 1. Borealis sensor for β-arrestin recruitment assists in demonstrating that the non-hallucinogenic 5-HT2A receptor (5-HT2AR) agonists lisuride and TBG, and IHCH-7079 can be distinguished from psychedelic drugs by low intrinsic efficacy rather than biased signaling in a recent study by Ippolito and colleagues10. Concentration-response curves of serotonin (5-HT) and a range of 5-HT2AR agonists at the (A) Gαq/11 signalling pathway using the inositol monophosphate (IP1) accumulation assay and (B) β-arrestin2 recruitment pathway using Montana Molecular’s Borealis fluorescent biosensor in recombinant human SH-SY5Y neuroblastoma cells. (C) 5-HT2AR agonist activity relative to serotonin for the IP1 accumulation and β-arrestin2 recruitment pathways, where deviation from the line of unity reflects biased signaling. Note that IHCH-7079 inhibited constitutive Gα q/11 signalling while producing β-arrestin recruitment, and as such, its bias for receptor activation was considered purely towards the latter pathway rather than quantified in relative terms.
This approach revealed that non-hallucinogenic analogues can be distinguished from psychedelic drugs on the basis of modest intrinsic efficacy rather than biased agonism between Gαq/11 signalling and β-arrestin recruitment. The former compounds produced considerably lower maximal responses in both signalling pathways (Figure 1A,B), whereas no clear pattern emerged for their bias (Figure 1C). This finding adds to the evidence for this mechanism controlling the hallucinogenic effects of 5-HT2AR agonists5, and aligns with similar results that have been demonstrated for drugs targeting other receptors where different grades of efficacy are required to achieve certain physiological outcomes and moderate efficacy can offer a greater safety profile. For instance, moderate intrinsic efficacy agonists of the µ-opioid receptor produce analgesia with limited respiratory depression17, and those of the GABAA receptor produce anxiolysis with limited sedation.30-36 Promisingly, non-hallucinogenic 5-HT2AR agonists have in some cases been shown to retain the therapeutic benefits of inducing neuroplasticity, producing antidepressant-like effects, and reducing drug-seeking behaviour in preclinical models6,9,37,38.
The work of Ippolito and colleagues, facilitated by the biosensor technology provided by Montana Molecular, paves the way for developing safer and more accessible treatments for psychiatric disorders based on the therapeutic potential of psychedelic drugs through efforts focusing on moderate intrinsic efficacy 5-HT2AR agonists.
References
- Roth, B. L. & Gumpper, R. H. Psychedelics as Transformative Therapeutics. Am J Psychiatry 180, 340-347 (2023). https://doi.org/10.1176/appi.ajp.20230172
- Peplow, M. Next-generation psychedelics: should new agents skip the trip? Nat Biotechnol 42, 827-830 (2024). https://doi.org/10.1038/s41587-024-02285-1
- Dolgin, E. Taking the tripping out of psychedelic medicine. Nature 609, S80-s82 (2022). https://doi.org/10.1038/d41586-022-02869-4
- McClure-Begley, T. D. & Roth, B. L. The promises and perils of psychedelic pharmacology for psychiatry. Nat Rev Drug Discov 21, 463-473 (2022). https://doi.org:10.1038/s41573-022-00421-7
- Wallach, J. et al. Identification of 5-HT2A receptor signaling pathways associated with psychedelic potential. Nat Commun 14, 8221 (2023). https://doi.org:10.1038/s41467-023-44016-1
- Pogorelov, V. M., Rodriguiz, R. M., Roth, B. L. & Wetsel, W. C. The G protein biased serotonin 5-HT2A receptor agonist lisuride exerts anti-depressant drug-like activities in mice. Front Mol Biosci 10, 1233743 (2023). https://doi.org/10.3389/fmolb.2023.1233743
- Rodriguiz, R. M. et al. LSD-stimulated behaviors in mice require β-arrestin 2 but not β-arrestin 1. Sci Rep 11, 17690 (2021). https://doi.org/10.1038/s41598-021-96736-3
- Kim, K. et al. Structure of a Hallucinogen-Activated Gq-Coupled 5-HT2A Serotonin Receptor. Cell 182, 1574-1588.e1519 (2020). https://doi.org/10.1016/j.cell.2020.08.024
- Cao, D. et al. Structure-based discovery of nonhallucinogenic psychedelic analogs. Science 375, 403-411 (2022). https://doi.org:10.1126/science.abl8615
- Ippolito, A. et al. Evidence that 5-HT2A receptor signalling efficacy and not biased agonism differentiates serotonergic psychedelic from non-psychedelic drugs. British Journal of Pharmacology, 182 1476-5381 (2025). https://doi.org/10.1101/2024.06.13.594677
- Hoare, S. R. J., Tewson, P. H., Quinn, A. M. & Hughes, T. E. A kinetic method for measuring agonist efficacy and ligand bias using high resolution biosensors and a kinetic data analysis framework. Scientific Reports 10, 1766 (2020). https://doi.org/10.1038/s41598-020-58421-9
- Hoare, S. R. J., Tewson, P. H., Quinn, A. M., Hughes, T. E. & Bridge, L. J. Analyzing kinetic signaling data for G-protein-coupled receptors. Scientific Reports 10, 12263 (2020). https://doi.org/10.1038/s41598-020-67844-3
- Hoare, S. R. J. et al. Quantifying the Kinetics of Signaling and Arrestin Recruitment by Nervous System G-Protein Coupled Receptors. Frontiers in Cellular Neuroscience 15 (2022). https://doi.org:10.3389/fncel.2021.814547
- Wright, S. C. et al. Conformation- and activation-based BRET sensors differentially report on GPCR-G protein coupling. Science signaling 17, eadi4747 (2024). https://doi.org/10.1126/scisignal.adi4747
- Gillis, A. et al. Critical Assessment of G Protein-Biased Agonism at the μ-Opioid Receptor. Trends in Pharmacological Sciences 41, 947-959 (2020). https://doi.org/10.1016/j.tips.2020.09.009
- Gillis, A., Sreenivasan, V. & Christie, M. J. Intrinsic Efficacy of Opioid Ligands and Its Importance for Apparent Bias, Operational Analysis, and Therapeutic Window. Molecular Pharmacology 98, 410 (2020). https://doi.org/10.1124/mol.119.119214
- Gillis, A. et al. Low intrinsic efficacy for G protein activation can explain the improved side effect profiles of new opioid agonists. Science signaling 13, eaaz3140 (2020). https://doi.org/10.1126/scisignal.aaz3140
- Kenakin, T. Biased Receptor Signaling in Drug Discovery. Pharmacol Rev 71, 267-315 (2019). https://doi.org/10.1124/pr.118.016790
- Kolb, P. et al. Community guidelines for GPCR ligand bias: IUPHAR review 32. British Journal of Pharmacology 179, 3651-3674 (2022). https://doi.org/10.1111/bph.15811
- Nagi, K. How can we improve the measurement of receptor signaling bias? Expert Opinion on Drug Discovery 18, 575-578 (2023). https://doi.org/10.1080/17460441.2023.2204225
- Klein Herenbrink, C. et al. The role of kinetic context in apparent biased agonism at GPCRs. Nat Commun 7, 10842 (2016). https://doi.org/10.1038/ncomms10842
- Gundry, J., Glenn, R., Alagesan, P. & Rajagopal, S. A Practical Guide to Approaching Biased Agonism at G Protein Coupled Receptors. Front Neurosci 11, 17 (2017). https://doi.org/10.3389/fnins.2017.00017
- Kenakin, T. & Christopoulos, A. Signalling bias in new drug discovery: detection, quantification and therapeutic impact. Nature Reviews Drug Discovery 12, 205-216 (2013). https://doi.org/10.1038/nrd3954
- Iwata, K. Studies of a Neuronal Cell Line as a Model of Psychiatric Disorders. Methods in molecular biology (Clifton, N.J.) 1735, 231-238 (2018). https://doi.org/10.1007/978-1-4939-7614-0_13
- Martín-Montañez, E., López-Téllez, J. F., Acevedo, M. J., Pavía, J. & Khan, Z. U. Efficiency of gene transfection reagents in NG108-15, SH-SY5Y and CHO-K1 cell lines. Methods Find Exp Clin Pharmacol 32, 291-297 (2010). https://doi.org/10.1358/mf.2010.32.5.1498327
- Rahimi, P. et al. Comparison of transfection efficiency of polymer-based and lipid-based transfection reagents. Bratisl Lek Listy 119, 701-705 (2018). https://doi.org/10.4149/bll_2018_125
- Chong, Z. X., Yeap, S. K. & Ho, W. Y. Transfection types, methods and strategies: a technical review. PeerJ 9, e11165 (2021). https://doi.org/10.7717/peerj.11165
- Washbourne, P. & McAllister, A. K. Techniques for gene transfer into neurons. Current Opinion in Neurobiology 12, 566-573 (2002). https://doi.org/10.1016/S0959-4388(02)00365-3
- Afzali, K. & Connor, M. Employing the Operational Model to Measure System-Independent OTR Efficacy. Methods in molecular biology (Clifton, N.J.) 2384, 201-220 (2022). https://doi.org/10.1007/978-1-0716-1759-5_12
- Jackson, H. C. Benzodiazepine partial agonists. J Psychopharmacol 7, 101-103 (1993). https://doi.org/10.1177/026988119300700119
- Haefely, W. et al. Partial agonists of benzodiazepine receptors for the treatment of epilepsy, sleep, and anxiety disorders. Adv Biochem Psychopharmacol 47, 379-394 (1992).
- Facklam, M. et al. Relationship between benzodiazepine receptor occupancy and functional effects in vivo of four ligands of differing intrinsic efficacies. J Pharmacol Exp Ther 261, 1113-1121 (1992).
- Haefely, W., Martin, J. R. & Schoch, P. Novel anxiolytics that act as partial agonists at benzodiazepine receptors. Trends Pharmacol Sci 11, 452-456 (1990). https://doi.org/10.1016/0165-6147(90)90126-s
- Basile, A. S., Lippa, A. S. & Skolnick, P. Anxioselective anxiolytics: can less be more? Eur J Pharmacol 500, 441-451 (2004). https://doi.org/10.1016/j.ejphar.2004.07.043
- Skolnick, P. Anxioselective anxiolytics: on a quest for the Holy Grail. Trends Pharmacol Sci 33, 611-620 (2012). https://doi.org/10.1016/j.tips.2012.08.003
- Sieghart, W. Anxioselective anxiolytics: additional perspective. Trends Pharmacol Sci 34, 145-146 (2013). https://doi.org/10.1016/j.tips.2013.01.005
- Lewis, V. et al. A non-hallucinogenic LSD analog with therapeutic potential for mood disorders. Cell Rep 42, 112203 (2023). https://doi.org/10.1016/j.celrep.2023.112203
- Cameron, L. P. et al. A non-hallucinogenic psychedelic analogue with therapeutic potential. Nature 589, 474-479 (2021). https://doi.org/10.1038/s41586-020-3008-z
