GPCR Biology
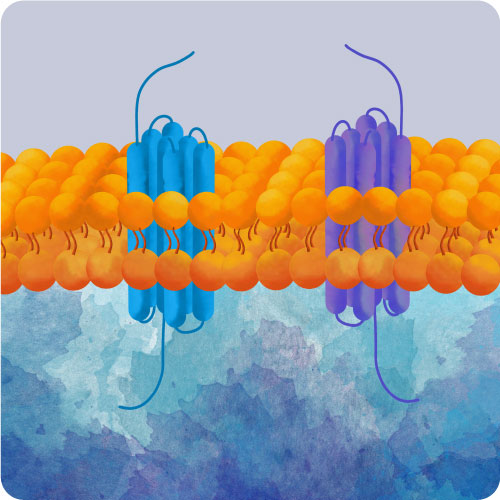
G-Protein Coupled Receptors (GPCRs) are one of the largest receptor families in the genome and are essential for the healthy function of nearly every organ in the body. GPCRs are also important targets for therapeutic drugs. Increase your understanding of drug effects and GPCR biology with bright fluorescent assays in living cells.
With our GPCR Assays in Living Cells, you can:
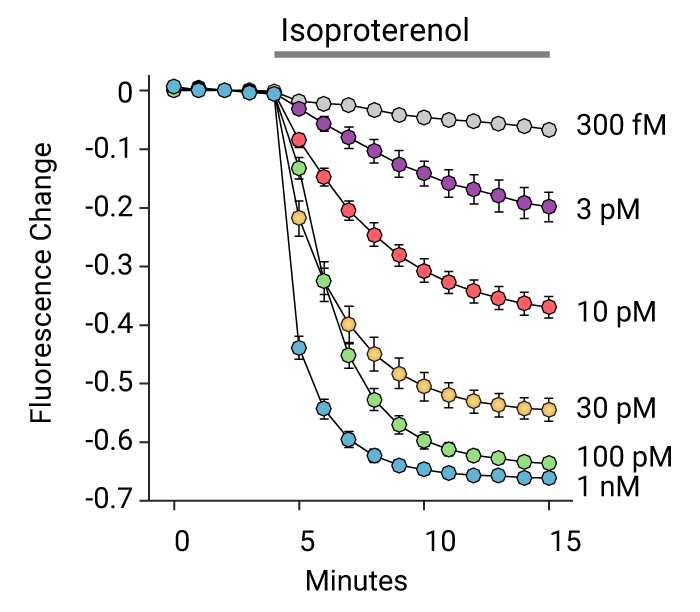
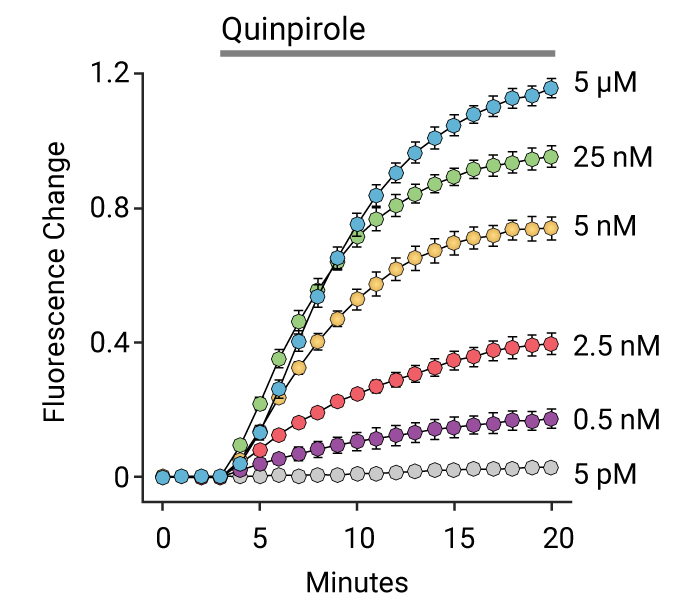
- Detect kinetic Gs, Gi, and Gq mediated responses in living cells
- Measure arrestin recruitment
- Combine multiple assays in the same cell population
- Express sensors and run assays in disease relevant cell types
- Detect fluorescence on imaging systems or automated plate readers (Z’ > 0.8)
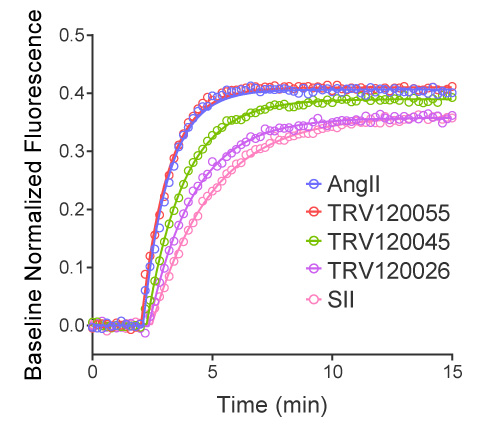
- Quantify agonist bias
- De-orphanize receptors
Detecting β-Arrestin
Fluorescent Borealis arrestin assays to detect arrestin recruitment at specific GPCRs.
Measuring GPCR Signaling Kinetics
See the Assay Guidance Manual chapter from NIH NCATS:
Express GPCRs with BacMam
We have a growing catalog of GPCRs packaged in a modified Baculovirus vector, BacMam. BacMam transduction is a low-cost, effective method to express GPCRs in your cells of interest, including in primary and iPSC derived cells. See low cell-to-cell variability in expression, get consistent results from experiment to experiment, and titrate expression to your desired level. For more information and a current list of available receptors please see our GPCRs page.
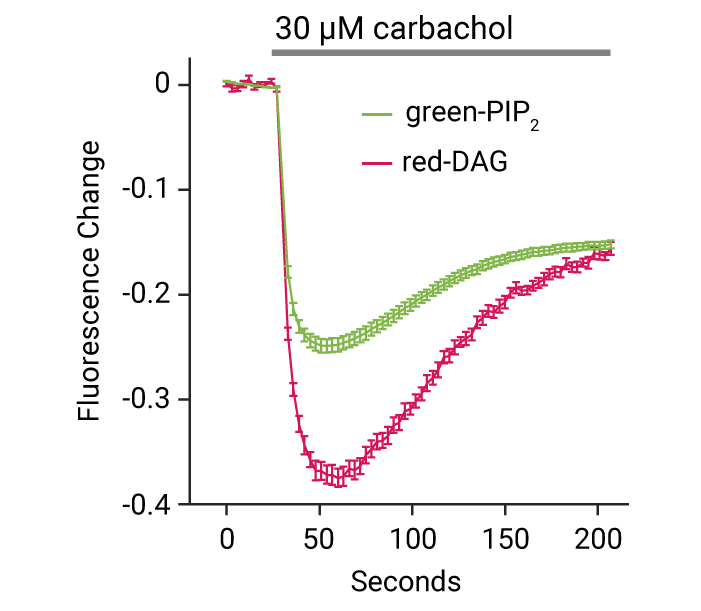
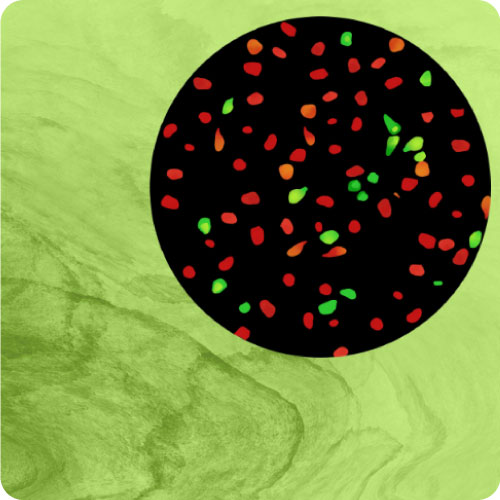
Continuous Multiplex Measurements
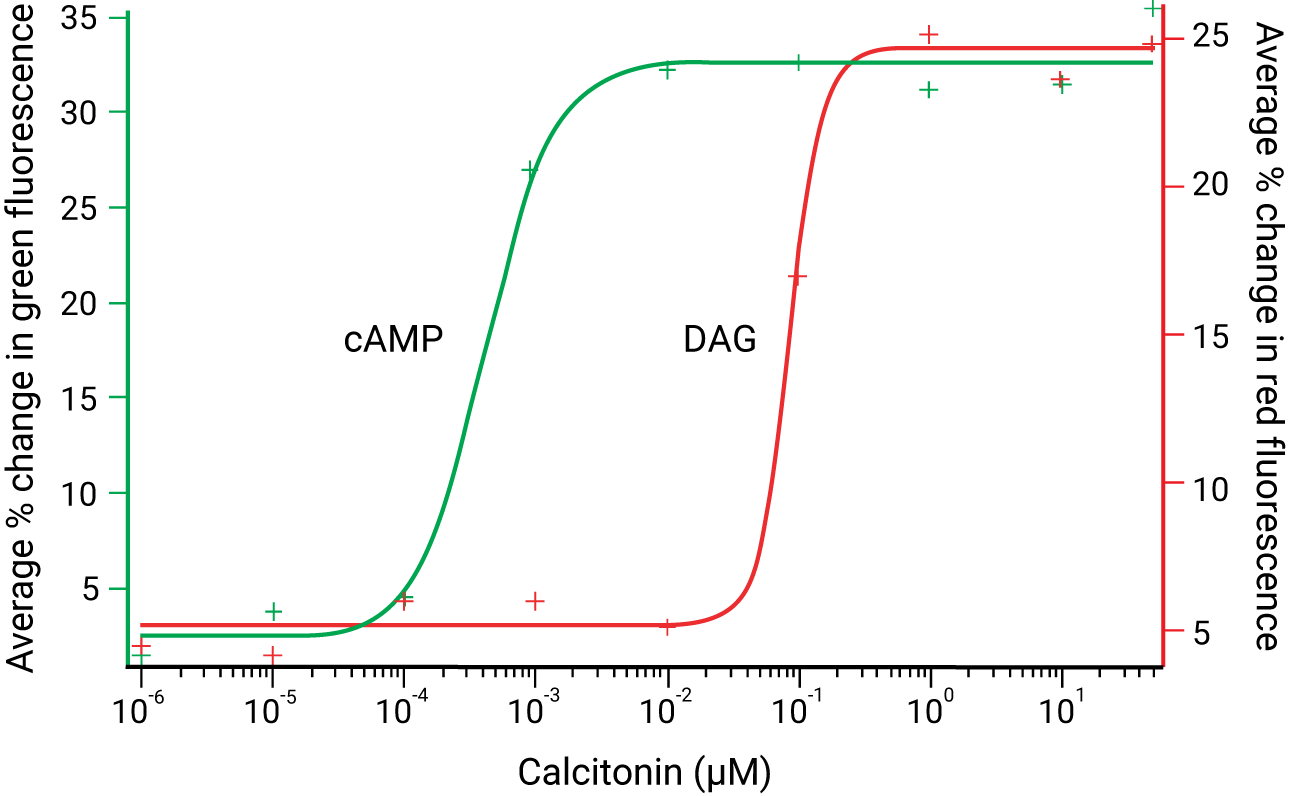
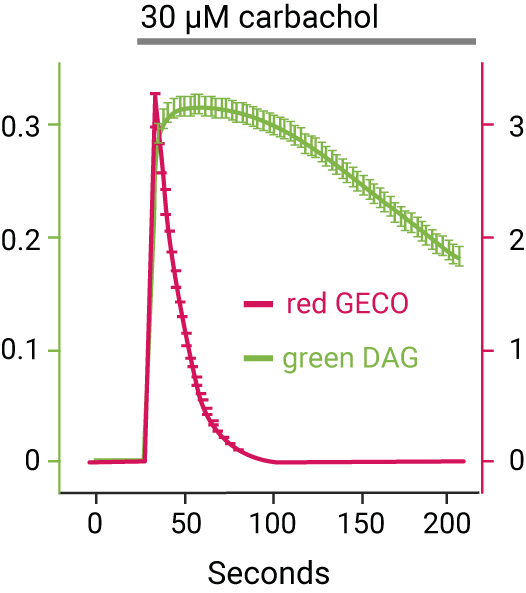
Red fluorescent DAG sensor multiplexed with cADDis, a green fluorescent cAMP sensor, indicates Gs & Gq signaling via a calcitonin receptor.
Assays in Primary Cultures or iPSCs
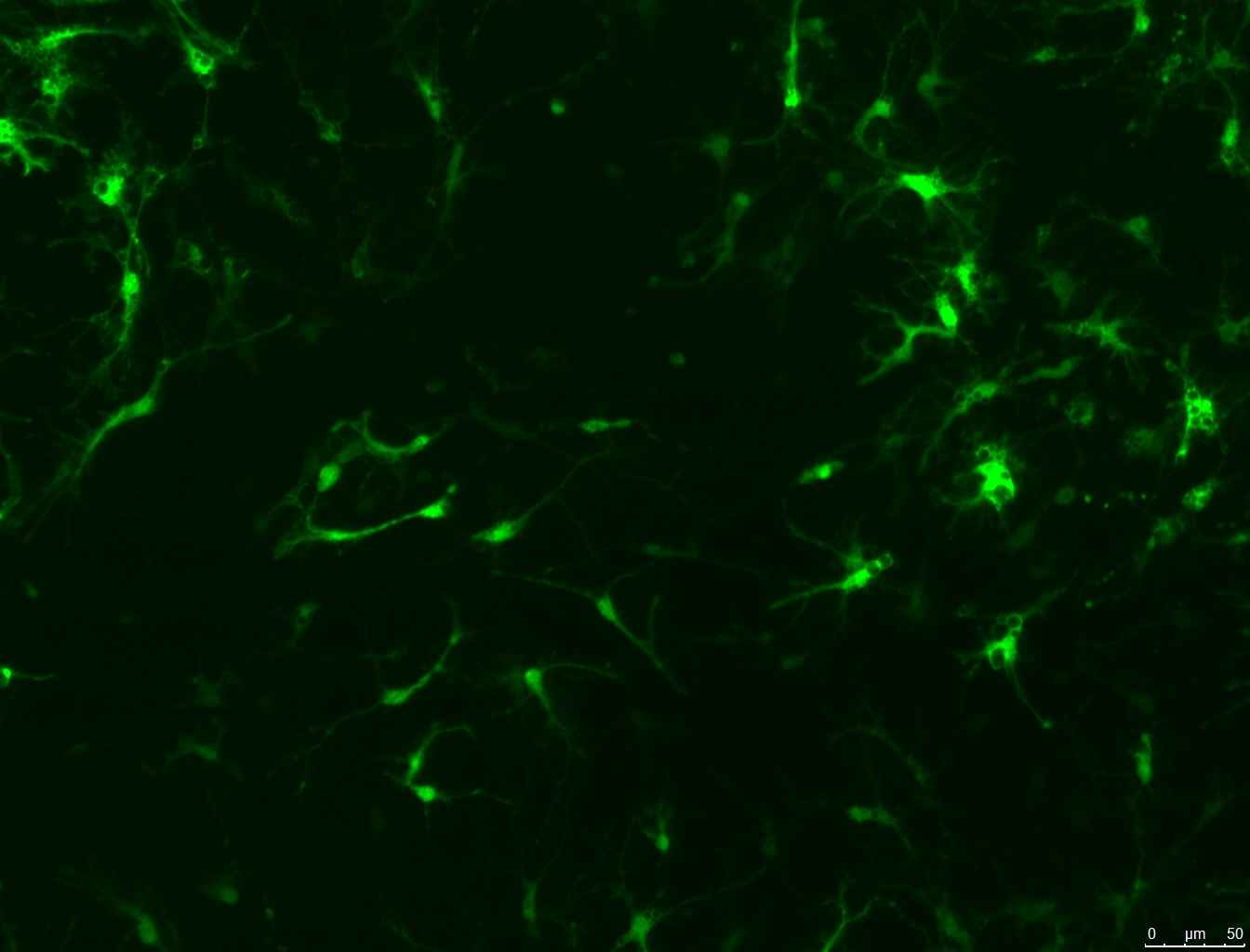
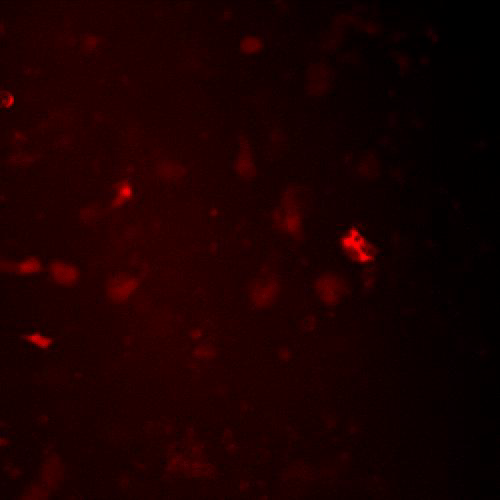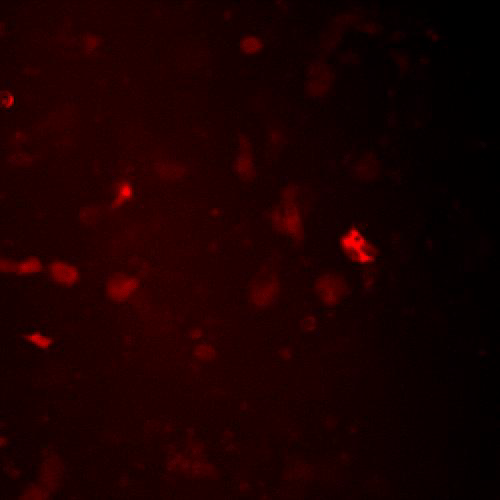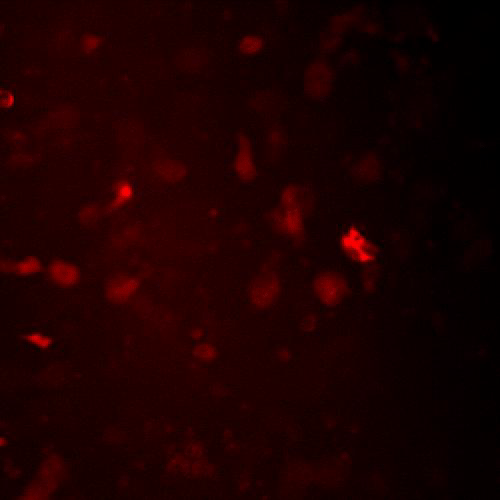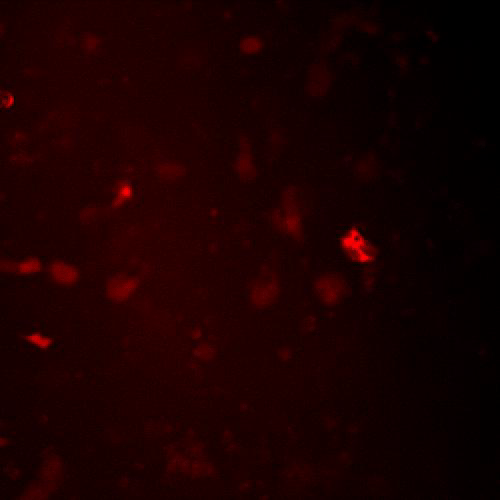
Detect GPCR mediated responses in cells relevant to your disease, drug target, or biology of interest. Our BacMam-packaged sensors have been used in neurons, cardiomyoctes, islets, and many more primary and iPSC derived cells. Examples can be found on our Scientific Publications page. Alternate promoters or viral vectors are available by request. Pictured, at right, are the cADDis cAMP Assay in primary striatal neurons and the Red GECO calcium assay in nCardia’s Cor.4u cardiomyocytes.
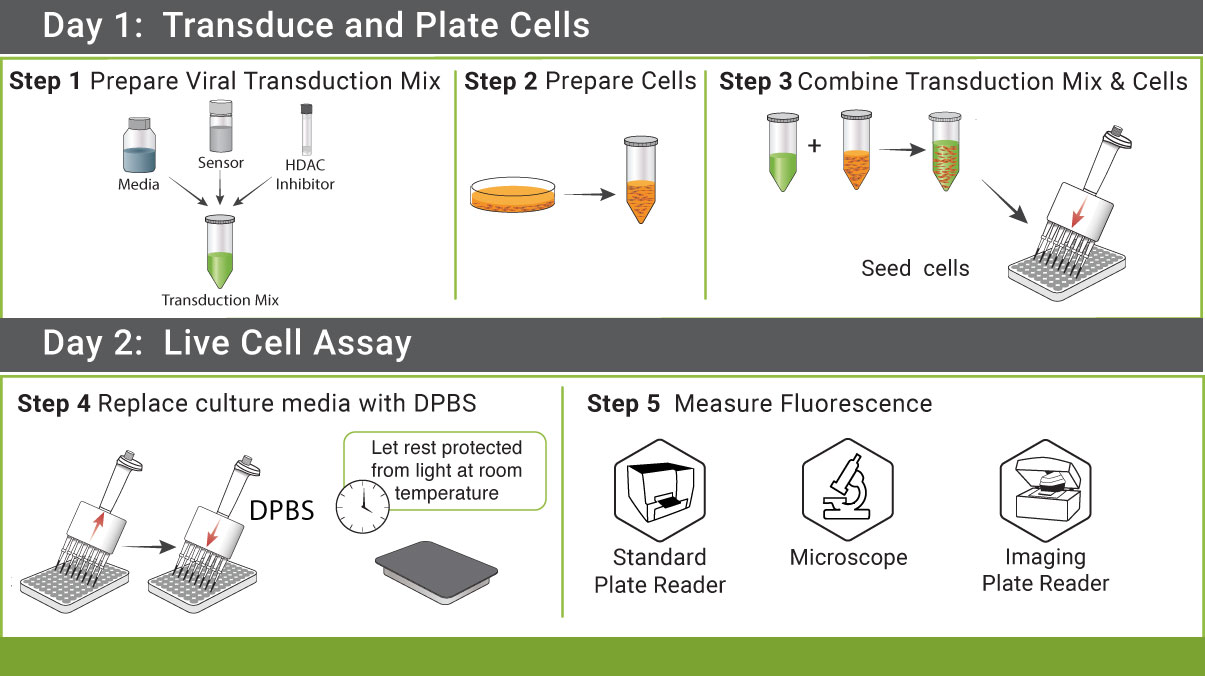
Simple Protocol
We strive to create simple protocols with minimal liquid handling. Cell lysis, IBMX, enzymes, and co-factors are not necessary for these assays. Add our sensors to your cells, incubate, add drug, and measure fluorescent changes.
Read more on each of our specific GPCR Assays and receptors:
Shop Other Assay Kits by Category
Recent Publications
GPCR Assay References
Borealis Arrestin Assay References
- A. Ippolito, et al. Increased 5-HT2A receptor signalling efficacy differentiates serotonergic psychedelics from non-psychedelics. bioRxiv. June 2024.
- H. Schiff, et al. β-arrestin-biased proteinase-activated receptor-2 antagonist C781 limits allergen-induced airway hyperresponsiveness and inflammation. British Journal of Pharmacology. June 2022.
- S. Hoare, et al. Quantifying the Kinetics of Signaling and Arrestin Recruitment by Nervous System G-Protein Coupled Receptors. Frontiers in Cellular Neuroscience. January 2022.
- S. Hoare, T. Hughes. Biosensor Assays for Measuring the Kinetics of G-Protein and Arrestin-Mediated Signaling in Live Cells. The Assay Guidance Manual. September 2021.
- S. Hoare, et al. Analyzing kinetic signaling data for G-protein-coupled receptors. Nature Scientific Reports. July 2020.
- S. Hoare, et al. A kinetic method for measuring agonist efficacy and ligand bias using high resolution biosensors and a kinetic data analysis framework. Nature Scientific Reports. Feb 2020.
cADDis cAMP Assay References
- S. Shi, et al. A high-affinity, cis-on photoswitchable beta blocker to optically control β2-adrenergic receptors in vitro and in vivo. Biochemical Pharmacology. June 2024.
- S. Ansari, et al. Sonic Hedgehog activates prostaglandin signaling to stabilize primary cilium length. Journal of Cell Biology. June 2024.
- A. Oqua, et al. Molecular mapping and functional validation of GLP-1R cholesterol binding sites in pancreatic beta cells. bioRxiv. June 2024.
- R. Schuck, et al. Cholesterol inhibits assembly and activation of the EphA2 receptor. bioRxiv. June 2024.
- X. Chen, et al. A PACAP-activated network for secretion requires coordination of Ca2+ influx and Ca2+ mobilization. Molecular Biology of the Cell. May 2024. (bioRxiv)
- L. Bridge, et al. Computational modelling of dynamic cAMP responses to GPCR agonists for exploration of GLP-1R ligand effects in pancreatic β-cells and neurons. Cellular Signaling, April 2024.
- R. Fagan, et al. Selective targeting of mu opioid receptors to primary cilia. Cell Reports. April 2024.
- G. Austin, et al. An inter-organelle contact between endosomal GLP-1R, ER VAP-B, and the mitochondrial AKAP SPHKAP triggers PKA-dependent MIC19 phosphorylation and β-cell mitochondrial remodelling. bioRxiv. April 2024.
- I. Cattani-Cavalieri, et al. Real-Time cAMP Dynamics in Live Cells Using the Fluorescent cAMP Difference Detector In Situ. Journal of Visualized Experiments. March 2024.
- B. Polacco, et al. Profiling the proximal proteome of the activated μ-opioid receptor. nature chemical biology. March 2024.
- P. Balraj, et al. Kisspeptin/KISS1R Signaling Modulates Human Airway Smooth Muscle Cell Migration. American Journal of Respiratory Cell and Molecular Biology. March 2024.
- J. Xu, et al. An evolutionarily conserved olfactory receptor is required for sex differences in blood pressure. Science Advances. March 2024 (bioRxiv)
- J. Alvarado et al. Transient cAMP production drives rapid and sustained spiking in brainstem parabrachial neurons to suppress feeding. Neuron. February 2024. (bioRxiv)
- H. Hamzeh, et al. Deciphering rapid cell signaling and control of cell motility by reverse opto-chemical engineering. bioRxiv. February 2024.
- N. Philip. Fatty acid metabolism promotes TRPV4 activity in lung microvascular endothelial cells in pulmonary arterial hypertension. Lung Cellular and Molecular Physiology. January 2024.
- L. Ripoll & M. von Zastrow. Spatial organization of adenylyl cyclase and its impact on dopamine signaling in neurons. bioRxiv. December 2023.
- T. Richardson, Y. Pettway, et al. Human Pseudoislet System for Synchronous Assessment of Fluorescent Biosensor Dynamics and Hormone Secretory Profiles. Journal of Visualized Experiments. November 2023.
- C. Hinds, et al. Abolishing β-arrestin recruitment is necessary for the full metabolic benefits of G protein-biased glucagon-like peptide-1 receptor agonists. Diabetes, Obesity and Metabolism. October 2023.
- E. Blythe & M. von Zastrow. β-Arrestin-
independent endosomal cAMP signaling by a polypeptide hormone GPCR. Nature Chemical Biology. September 2023. - R. Matt, et al. Fingerprinting heterocellular β-adrenoceptor functional expression in the brain using agonist activity profiles. Frontiers in Molecular Biosciences. August 2023.
- Y. Kunioku, et al. Intracellular cAMP Signaling Pathway via Gs Protein-Coupled Receptor Activation in Rat Primary Cultured Trigeminal Ganglion Cells. Biomedicines. August 2023.
- K.M. Semesta, et al. The psychosis risk factor RBM12 encodes a novel repressor of GPCR/cAMP signal transduction. Journal of Biological Chemistry. August 2023.
- Y.J. Peng, et al. Hypoxia sensing requires H2S-dependent persulfidation of olfactory receptor 78. Science Advances. July 2023.
- S. Zhang, et al. Competition between stochastic neuropeptide signals calibrates the rate of satiation. bioRxiv. July 2023.
DAG Assay References
- X. Chen, et al. A PACAP-activated network for secretion requires coordination of Ca2+ influx and Ca2+ mobilization. Molecular Biology of the Cell. May 2024. (bioRxiv)
- S. Datta, et al. APOL1-mediated monovalent cation transport contributes to APOL1-mediated podocytopathy in kidney disease. The Journal of Clinical Investigation. January 2024. (Supplemental Data)
- Z. Miller, et al. Lidocaine induces apoptosis in head and neck squamous cell carcinoma through activation of bitter taste receptor T2R14. Cell Reports. November 2023. (bioRxiv)
- N. Zaïmia, et al. GLP-1 and GIP receptors signal through distinct β-arrestin 2-dependent pathways to regulate pancreatic β cell function. Cell Reports. October 2023.
- G. Sanchez, et al. Coincident Regulation of PLCβ Signaling by Gq-Coupled and μ-Opioid Receptors Opposes Opioid-Mediated Antinociception. Molecular Pharmacology. December 2022.
- Z. Miller, et al. Lidocaine Induces Apoptosis in Head and Neck Squamous Cell Carcinoma Cells Through Activation of Bitter Taste Receptor T2R14. bioRxiv. November 2022.
- M. Doepner, et al. Endogenous DOPA inhibits melanoma through suppression of CHRM1 signaling. Science Advances. September 2022.
PIP2 Assay References
- L. Brueggemann, et al. Structural Determinants of Kv7.5 Potassium Channels that Confer Changes in Phosphatidylinositol 4,5-Biphosphate (PIP2) Affinity and Signaling Sensitivity in Smooth Muscle Cells. Molecular Pharmacology. March 2020.
- L.Liu, et al. Gαq sensitizes TRPM8 to inhibition by PI(4,5)P2 depletion upon receptor activation. J.Neurosci. May 2019.
- , and Teaching an Old Drug New Tricks: Agonism, Antagonism, and Biased Signaling of Pilocarpine through M3 Muscarinic Acetylcholine Receptor Molecular Pharmacology Nov. 2018
GECO Calcium Assay References
- X. Chen, et al. A PACAP-activated network for secretion requires coordination of Ca2+ influx and Ca2+ mobilization. Molecular Biology of the Cell. May 2024. (bioRxiv)
- C. Amos, et al. Membrane lipids couple synaptotagmin to SNARE-mediated granule fusion in insulin-secreting cells. Molecular Biology of the Cell. December 2023.
- J. Wu, et al. Interaction Between HCN and Slack Channels Regulates mPFC Pyramidal Cell Excitability and Working Memory. bioRxiv. March 2023.
- M. Thomas, et al. Optically activated, customizable, excitable cells. PLOS One. December 2020.
- L. Liu, et al. Diacylglycerol kinases regulate TRPV1 channel activity. Journal of Biological Chemistry. April 2020.
- S. Hoare, et al. A kinetic method for measuring agonist efficacy and ligand bias using high resolution biosensors and a kinetic data analysis framework. Nature Scientific Reports Feb 2020.
- K. Harlen, et al. Live-Cell Assays for Cell Stress Responses Reveal New Patterns of Cell Signaling Caused by Mutations in Rhodopsin, α-Synuclein and TDP-43 Front. Cell. Neurosci.,December 2019