PDE Biology
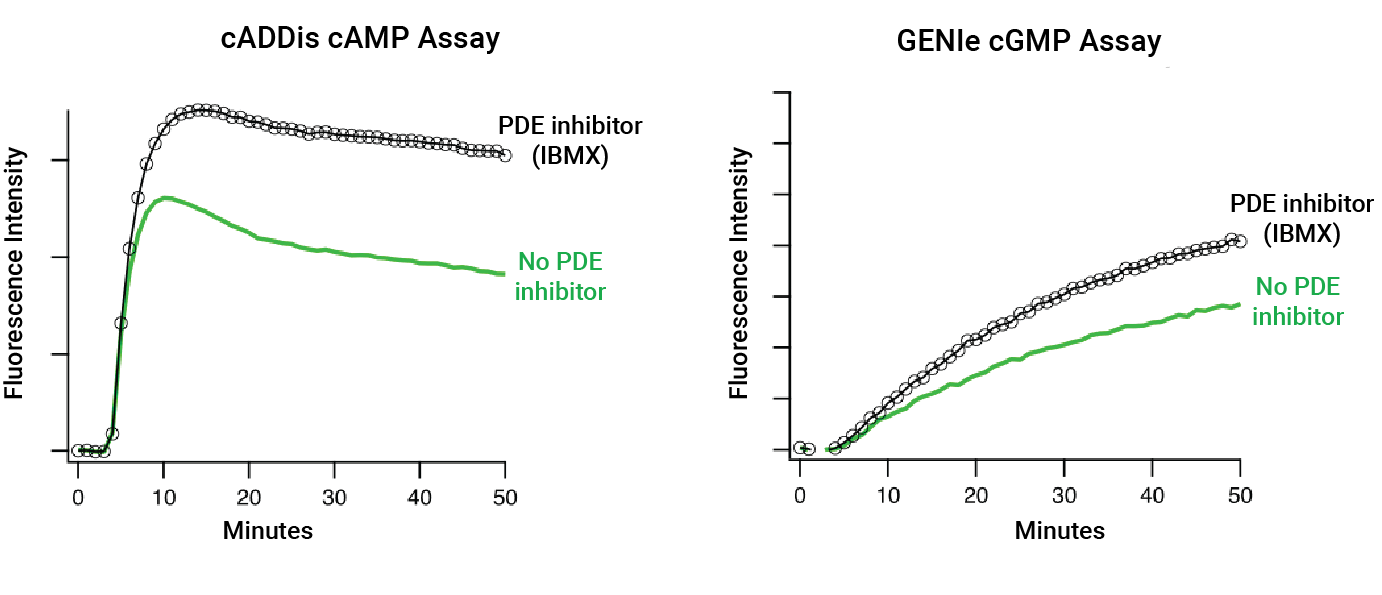
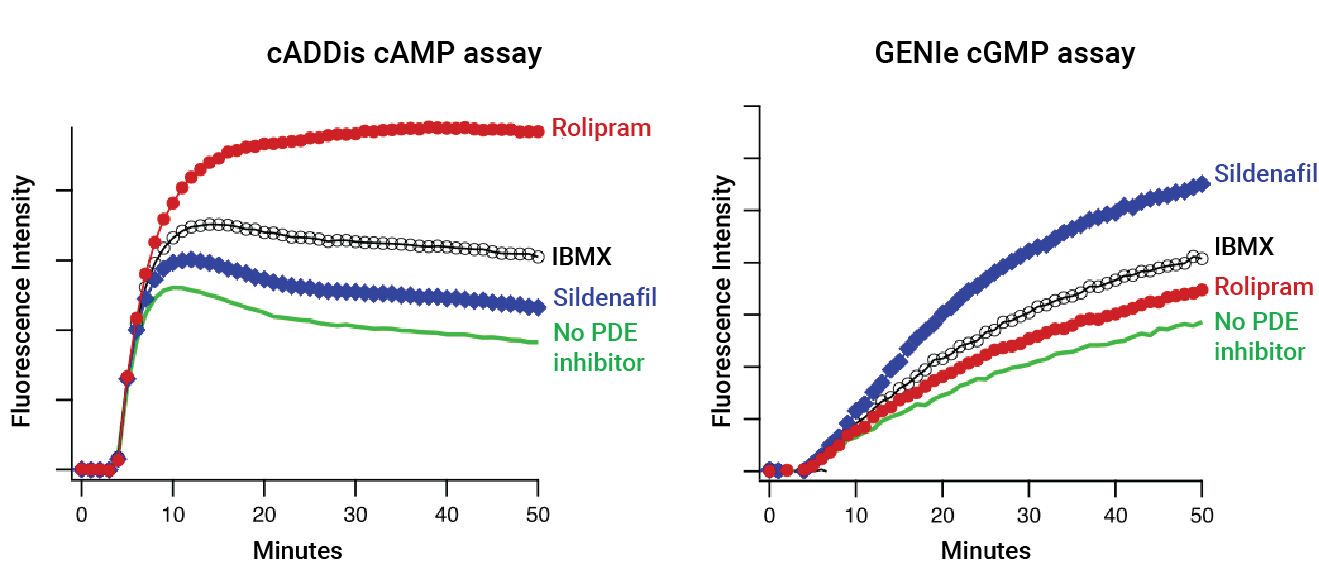
Cyclic nucleotide phosphodiesterases (PDEs) are important drug targets with unique tissue distribution and functional properties. This family of enzymes is key in the regulation of cyclic nucleotide second messenger levels because they degrade both cAMP and cGMP. Some PDEs hydrolyze both cAMP and cGMP, while others are either cAMP or cGMP hydrolases. Selective PDE inhibitors block the activity of specific PDEs and are used to treat a variety of diseases. Non-selective PDE inhibitors include IBMX and caffeine, both of which are important consumables in basic research laboratories.
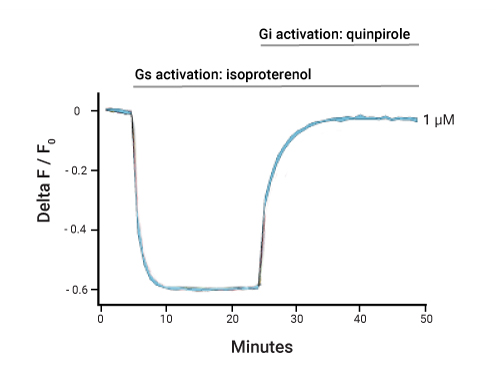
Detecting cAMP
cAMP response to isoproterenol followed by inhibition of cAMP production with quinpirole. Detected with the cADDis cAMP assay.
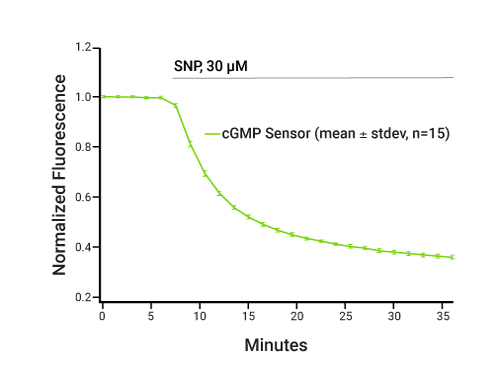
PDE Selectivity Assay
An Optogenetic Tool for Monitoring PDE Rates
The blue-light activated adenylyl cyclase bPAC (#V0100N) can be co-expressed with R-cADDis (#U0200R), a red fluorescent biosensor for cAMP. A two second pulse of blue light activates bPAC to stimulate cAMP production. cAMP degradation by PDE is monitored in real time using R-cADDis. Read about how this method can be used to study the role of PDE activity in neurodegeneration: Live-Cell Assays for Cell Stress Responses Reveal New Patterns of Cell Signaling Caused by Mutations in Rhodopsin, α-Synuclein and TDP-43.

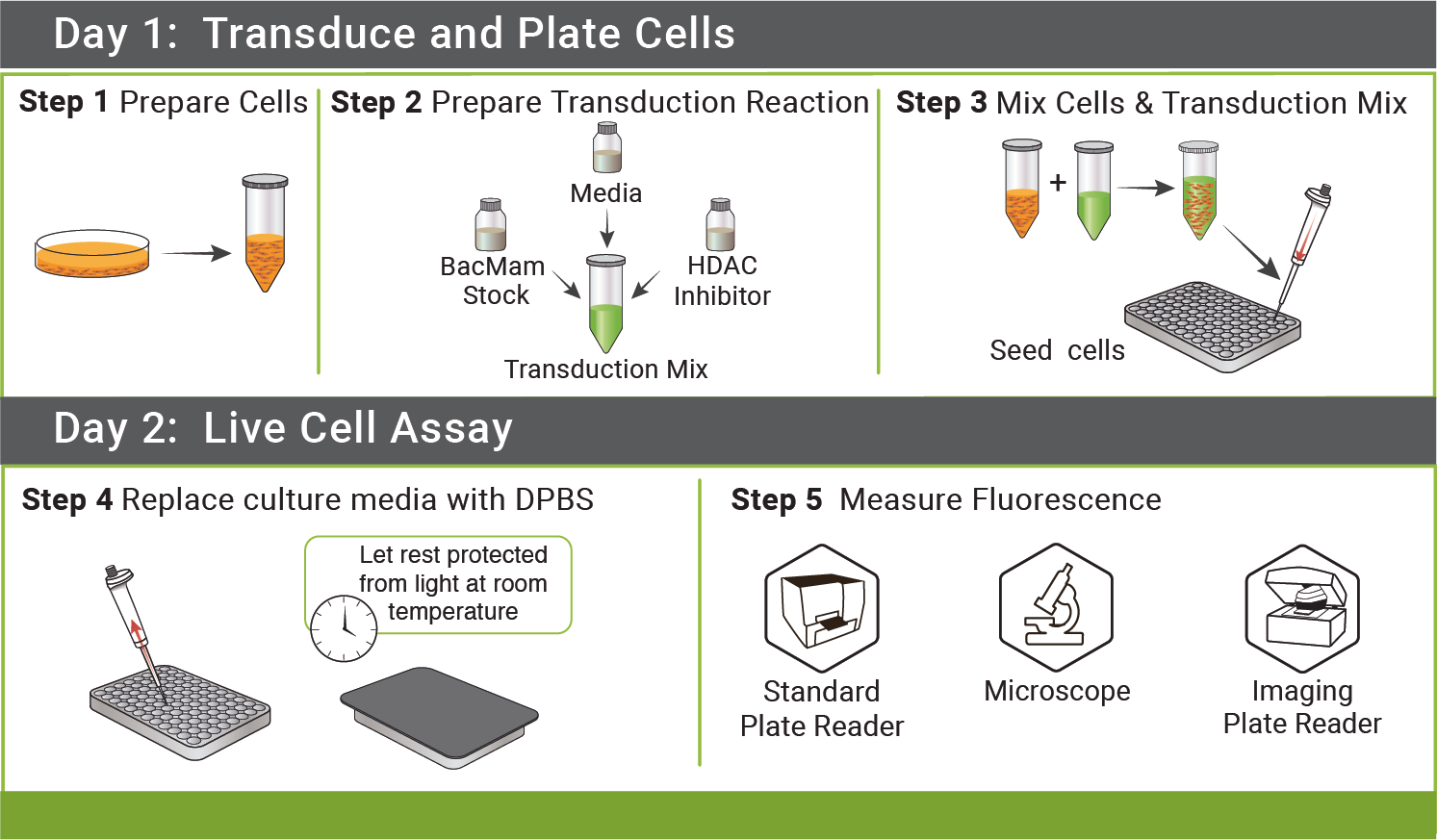
Simple Protocol
Simple, step-by-step protocols for optimizing cAMP and cGMP assays are available online. These assays are non-FRET and cell lysis, enzymes, and co-factors are not necessary. Add our sensors to your cells, incubate, add drug, and measure fluorescence changes.
Sound Intriguing? Get more details on our PDE Biology Assays:
cADDis cAMP Assay
GENIe cGMP Assay
bPAC in BacMam
Recent Publications
- S. Shi, et al. A high-affinity, cis-on photoswitchable beta blocker to optically control β2-adrenergic receptors in vitro and in vivo. Biochemical Pharmacology. June 2024.
- S. Ansari, et al. Sonic Hedgehog activates prostaglandin signaling to stabilize primary cilium length. Journal of Cell Biology. June 2024.
- A. Oqua, et al. Molecular mapping and functional validation of GLP-1R cholesterol binding sites in pancreatic beta cells. bioRxiv. June 2024.
- R. Schuck, et al. Cholesterol inhibits assembly and activation of the EphA2 receptor. bioRxiv. June 2024.
- X. Chen, et al. A PACAP-activated network for secretion requires coordination of Ca2+ influx and Ca2+ mobilization. Molecular Biology of the Cell. May 2024. (bioRxiv)
- L. Bridge, et al. Computational modelling of dynamic cAMP responses to GPCR agonists for exploration of GLP-1R ligand effects in pancreatic β-cells and neurons. Cellular Signaling, April 2024.
- R. Fagan, et al. Selective targeting of mu opioid receptors to primary cilia. Cell Reports. April 2024.
- G. Austin, et al. An inter-organelle contact between endosomal GLP-1R, ER VAP-B, and the mitochondrial AKAP SPHKAP triggers PKA-dependent MIC19 phosphorylation and β-cell mitochondrial remodelling. bioRxiv. April 2024.
- I. Cattani-Cavalieri, et al. Real-Time cAMP Dynamics in Live Cells Using the Fluorescent cAMP Difference Detector In Situ. Journal of Visualized Experiments. March 2024.
- B. Polacco, et al. Profiling the proximal proteome of the activated μ-opioid receptor. nature chemical biology. March 2024.
- P. Balraj, et al. Kisspeptin/KISS1R Signaling Modulates Human Airway Smooth Muscle Cell Migration. American Journal of Respiratory Cell and Molecular Biology. March 2024.
- J. Xu, et al. An evolutionarily conserved olfactory receptor is required for sex differences in blood pressure. Science Advances. March 2024 (bioRxiv)
- J. Alvarado et al. Transient cAMP production drives rapid and sustained spiking in brainstem parabrachial neurons to suppress feeding. Neuron. February 2024. (bioRxiv)
- H. Hamzeh, et al. Deciphering rapid cell signaling and control of cell motility by reverse opto-chemical engineering. bioRxiv. February 2024.
- N. Philip. Fatty acid metabolism promotes TRPV4 activity in lung microvascular endothelial cells in pulmonary arterial hypertension. Lung Cellular and Molecular Physiology. January 2024.
- X. Chen, et al. A PACAP-activated network for secretion requires coordination of Ca2+ influx and Ca2+ mobilization. bioRxiv. January 2024.
- L. Ripoll & M. von Zastrow. Spatial organization of adenylyl cyclase and its impact on dopamine signaling in neurons. bioRxiv. December 2023.
- T. Richardson, Y. Pettway, et al. Human Pseudoislet System for Synchronous Assessment of Fluorescent Biosensor Dynamics and Hormone Secretory Profiles. Journal of Visualized Experiments. November 2023.
- C. Hinds, et al. Abolishing β-arrestin recruitment is necessary for the full metabolic benefits of G protein-biased glucagon-like peptide-1 receptor agonists. Diabetes, Obesity and Metabolism. October 2023.
- E. Blythe & M. von Zastrow. β-Arrestin-
independent endosomal cAMP signaling by a polypeptide hormone GPCR. Nature Chemical Biology. September 2023. - R. Matt, et al. Fingerprinting heterocellular β-adrenoceptor functional expression in the brain using agonist activity profiles. Frontiers in Molecular Biosciences. August 2023.
- Y. Kunioku, et al. Intracellular cAMP Signaling Pathway via Gs Protein-Coupled Receptor Activation in Rat Primary Cultured Trigeminal Ganglion Cells. Biomedicines. August 2023.
- K.M. Semesta, et al. The psychosis risk factor RBM12 encodes a novel repressor of GPCR/cAMP signal transduction. Journal of Biological Chemistry. August 2023.
- Y.J. Peng, et al. Hypoxia sensing requires H2S-dependent persulfidation of olfactory receptor 78. Science Advances. July 2023.
- S. Zhang, et al. Competition between stochastic neuropeptide signals calibrates the rate of satiation. bioRxiv. July 2023.
- E. Kitayama, et al. Functional Expression of IP, 5-HT4, D1, A2A, and VIP Receptors in Human Odontoblast Cell Line. Biomolecules. May 2023.
- D. Santana Nunez, et al. Piezo1 induces endothelial responses to shear stress via soluble adenylyl Cyclase-IP3R2 circuit. iScience. May 2023.
- S. Bitsi, et al. Divergent acute versus prolonged pharmacological GLP-1R responses in adult β cell–specific β-arrestin 2 knockout mice. Science Advances. May 2023.
- V. Bhatia, et al. Characterization of Adenylyl Cyclase Isoform 6 Residues Interacting with Forskolin. Biology. April 2023.
- H. Carr, et al. The Wnt pathway protein Dvl1 targets Somatostatin receptor 2 for lysosome-dependent degradation. Journal of Biological Chemistry. March 2023.
- I. Cattani-Cavalieri, et al. Quantitative phosphoproteomic analysis reveals unique cAMP signaling pools emanating from AC2 and AC6 in human airway smooth muscle cells. Frontiers in Physiology. February 2023.
- E. Porpiglia, et al. Elevated CD47 is a hallmark of dysfunctional aged muscle stem cells that can be targeted to augment regeneration. Cell Stem Cell. December 2022. (bioRxiv)
- J. Janetzko, et al. Membrane phosphoinositides regulate GPCR-β-arrestin complex assembly and dynamics. Cell. November 2022.
- N. Saito, et al. Gαs-Coupled CGRP Receptor Signaling Axis from the Trigeminal Ganglion Neuron to Odontoblast Negatively Regulates Dentin Mineralization. biomolecules. November 2022.
- B. Barsi-Rhyne, et al. Discrete GPCR-triggered endocytic modes enable β-arrestins to flexibly regulate cell signaling. eLife. October 2022. (bioRxiv)
- S. A. Dai, et al. State-selective modulation of heterotrimeric Gαs signaling with macrocyclic peptides. Cell. September 2022.
- J. H. Cho, et al. Islet primary cilia motility controls insulin secretion. Science Advances. September 2022. (bioRxiv)
- E. Blythe, M. von Zastrow. A discrete mode of endosomal GPCR signaling that does not require β-arrestins. bioRxiv. September 2022.
- ER McGlone, et al. Hepatocyte cholesterol content modulates glucagon receptor signaling. Molecular Metabolism. September 2022.
- J. Xu, J. Pluznick. Key amino acids alter activity and trafficking of a well-conserved olfactory receptor. Cell Physiology. June 2022.
- C. Zhang, et al. A brainstem circuit for nausea suppression. Cell Reports. June 2022.
- J. Hansen, et al. A cAMP signalosome in primary cilia drives gene expression and kidney cyst formation. EMBO Reports. June 2022.
- S. Ansari, et al. Morphogen Directed Coordination of GPCR Activity Promotes Primary Cilium Function for Downstream Signaling. bioRxiv. May 2022.
- S. Bitsi, et al. Divergent acute versus prolonged in vivo GLP-1R responses in β-arrestin 2-deleted primary beta cells. bioRxiv. April 2022.
- Y. Mizobuchi, et al. Ketamine Improves Desensitization of μ-Opioid Receptors Induced by Repeated Treatment with Fentanyl but Not with Morphine. biomolecules. March 2022.
- B. Polacco, et al. Profiling the diversity of agonist-selective effects on the proximal proteome environment of G protein-coupled receptors. bioRxiv. March 2022.
- D. Lovinger, et al. Local modulation by presynaptic receptors controls neuronal communication and behavior. Nature Reviews Neuroscience. February 2022.
- F. De Logu, et al. Schwann cell endosome CGRP signals elicit peri orbital mechanical allodynia in mice. Nature Communications. February 2022.
- A. Lutas, et al. History-dependent dopamine release increases cAMP levels in most basal amygdala glutamatergic neurons to control learning. Cell Reports. January 2022.
- G. Sancar, et al. FGF1 and insulin control lipolysis by convergent pathways. Cell Metabolism. January 2022.
- S. Hoare, et al. Quantifying the Kinetics of Signaling and Arrestin Recruitment by Nervous System G-Protein Coupled Receptors. Frontiers in Cellular Neuroscience. January 2022.
- Y. Karasawa, et al. In Vitro Analyses of Spinach-Derived Opioid Peptides, Rubiscolins: Receptor Selectivity and Intracellular Activities through G-protein- and β-arrestin-Mediated Pathways. Molecules. October 2021.
- A. White, et al. Spatial Bias in cAMP generation determines biological responses to PTH type 1 receptor activation. Science Signaling. October 2021.
- J. Janetzko, et al. Membrane phosphoinositides stabilize GPCR-arrestin complexes and offer temporal control of complex assembly and dynamics. bioRxiv. October 2021.
- S. Hoare, T. Hughes. Biosensor Assays for Measuring the Kinetics of G-Protein and Arrestin-Mediated Signaling in Live Cells. The Assay Guidance Manual. September 2021.
- Y. Kuroda, et al. Inhibition of endothelin A receptor by a novel, selective receptor antagonist enhances morphine-induced analgesia: Possible functional interaction of dimerized endothelin A and μ-opioid receptors. Biomedicine & Pharmacotherapy. September 2021.
- A. Lutas, et al. Dopamine-dependent cAMP dynamics in basal amygdala glutamatergic neurons. bioRxiv. September 2021.
- F. De Logu, et al. CGRP Signals from Endosomes of Schwann Cells to Elicit Migraine Pain. Research Square. August 2021.
- C. Wu, et al. Discovery of ciliary G protein-coupled receptors regulating pancreatic islet insulin and glucagon secretion. Genes & Development. August 2021. bioRxiv.
- S. Zhang, et al. Hypothalamic dopamine neurons motivate mating through persistent cAMP signalling. Nature. August 2021.
- M. Nuriya, et al. Alkyne-Tagged Dopamines as Versatile Analogue Probes for Dopaminergic System Analysis. Analytical Chemistry. July 2021.
- A. Gad, et al. Conserved residues in extracellular loop 2 regulate Stachel-mediated activation of ADGRG2. Scientific Reports. July 2021. (bioRxiv)
- N. Senese, et al. Antidepressants produce persistent Gαs associated signaling changes in lipid rafts following drug withdrawal. Molecular Pharmacology. May 2021.
- W. Han, et al. Nausea and the Brain: The Chemoreceptor Trigger Zone Enters the Molecular Age. Neuron. February 2021.
- H. Carr, et al. The PDZ Domain Protein SYNJ2BP Regulates GRK-dependent Sst2A Phosphorylation and Downstream MAPK Signaling. Endocrinology. February 2021.
- A. Pronin, et al. Ectopically expressed olfactory receptors OR51E1 and OR51E2 suppress proliferation and promote cell death in a prostate cancer cell line. Journal of Biological Chemistry. February 2021.
- S. Thornquist, et al. Biochemical evidence accumulates across neurons to drive a network-level eruption. Molecular Cell. February 2021.
- Z. Zhou, et al. Astrocytic cAMP modulates memory via synaptic plasticity. PNAS. January 2021.
- M. Draper, et al. Imaging Meets Cytometry: Analyzing Heterogenous Functional Microscopic Data from Living Cell Populations. J. Imaging. January 2021.
- M. Thomas, et al. Optically activated, customizable, excitable cells. PLOS One. December 2020.
- C. Zhang, et al. Area Postrema Cell Types That Mediate Nausea-Associated Behaviors. Neuron. November 2020.
- T. Patriarchi, et al. An expanded palette of dopamine sensors for multiplex imaging in vivo. Nature Methods. September 2020.
- S. Hoare, et al. Analyzing kinetic signaling data for G-protein-coupled receptors. Nature Scientific Reports July 2020.
- J. Hansen, et al. Nanobody-directed targeting of ontogenetic tools to study signaling in the primary cilium. eLife. June 2020.
- J. Yu et al. N-3 polyunsaturated fatty acids promote astrocyte differentiation and neurotrophin production independent of cAMP in patient-derived neural stem cells. Mol. Psychiatry. June 2020.
- J. Cortes-Troncoso, et al. T cell exosome-derived miR-142-3p impairs glandular cell function in Sjörgen's Syndrome. JCI Insight. May 2020.
- I. Gapallawa, et al. Bitter taste receptors stimulate phagocytosis in human macrophages through calcium, nitric oxide, and cyclic-GMP signaling Cell. Mol. Life Sci. March, 2020 (bioRxiv)
- N. Daram, et al. Glucagon-like Peptide 1 Shows Glucose Dependence in Regulating Islet Hormone Secretion. Metabolism. March 2020.
- C. Koziol-White et al. Budesonide enhances agonist-induced bronchodilation in human small airways by increasing cAMP production in airway smooth muscle. American Journal of Physiology. February 2020.
- S. Hoare, et al. A kinetic method for measuring agonist efficacy and ligand bias using high resolution biosensors and a kinetic data analysis framework. Nature Scientific Reports Feb 2020.
- D. McMahon, et al. Neuropeptide regulation of secretion and inflammation in human airway gland serous cells (bioRxiv)
- R. Forteza, et al. Glucocorticoids and myosin5b loss of function induce heightened PKA signaling in addition to membrane traffic defects Molecular Biology of the Cell. Dec. 2019
- Nunez, F.J., Johnstone, T.B. et al. Glucocorticoids rapidly activate cAMP production via Gαs to initiate non‐genomic signaling that contributes to one‐third of their canonical genomic effects FASEB Journal, Dec. 2019
- Nunez, F.J., Schulte, N.A., Fogel, D.M., et al. Agonist-specific desensitization of PGE2-stimulated cAMP signaling due to upregulated phosphodiesterase expression in human lung fibroblasts Naunyn-Schmiedeberg’s Arch Pharmacol Dec. 2019
- K. Harlen, et al. Live-Cell Assays for Cell Stress Responses Reveal New Patterns of Cell Signaling Caused by Mutations in Rhodopsin, α-Synuclein and TDP-43 Front. Cell. Neurosci.,December 2019
- MP Pedro et al. Activation of G-Protein Coupled Receptor-Gai Signaling Increases Keratinocyte Proliferation and Reduces Differentiation, Leading to Epidermal Hyperplasia. Journal of Investigative Dermatology. November 2019.
- K. Hilgendorf, et al. Omega-3 Fatty Acids Activate Ciliary FFAR4 to Control Adipogenesis Cell November, 2019
- R. Sherpa et al. Sensory primary cilium is a responsive cAMP microdomain in renal epithelia. Scientific Reports. April 2019
- T. Baldwin, et al. Insights into the Regulatory Properties of Human Adenylyl Cyclase Type 9 Molecular Pharmacology. April, 2019
- T. Togo Autocrine purinergic signaling stimulated by cell membrane disruption is involved in both cell membrane repair and adaptive response in MDCK cells Biochem & Biophysical Research Comm. March, 2019
- J. Knudsen, et al. Dysregulation of Glucagon Secretion by Hyperglycemia-Induced Sodium-Dependent Reduction of ATP Production. Cell Metabolism. February 2019.
- C. Ojiaku, et al. Transforming Growth Factor-β1 Decreases β2-Agonist–induced Relaxation in Human Airway Smooth Muscle ATSJournas, Dec 2018
- N. Naim et al. Luminescence-activated nucleotide cyclase regulates spatial and temporal cAMP synthesis. Journal of Biological Chemistry. December 2018.
- Y.A.B. Sierra, et al. Potassium channel-based optogenetic silencing. Nature Communications Nov. 5 2018.
- M. Qadri et al. cAMP attenuates TGF-β’s profibrotic responses in osteoarthritic synoviocytes: involvement of hyaluronan and PRG4. Cell Physiology. September 2018.
- M. Marasco et al. Interleukin-6 Reduces Beta-Cell Oxidative Stress by Linking Autophagy With the Antioxidant Response. Diabetes. August 2018
- X. Chen, et al. Phenylephrine, a common cold remedy active ingredient, suppresses uterine contractions through cAMP signalling. Scientific Reports Aug. 2018.
- P. Tewson, et al. Assay for Detecting Gαi-Mediated Decreases in cAMP in Living Cells. SLAS. July 10, 2018. Request Full Text PDF
- A. Hamilton et al. Adrenaline Stimulates Glucagon Secretion by Tpc2-Dependent Ca2+ Mobilization From Acidic Stores in Pancreatic α-Cells. Diabetes. June 2018.
- T. Buranda, et al. A High-Throughput Flow Cytometry Screen Identifies Molecules that Inhibit Hantavirus Cell Entry. SLAS Discovery. April 2, 2018.
- N. H. Wray, et al. NMDAR-independent, cAMP-dependent antidepressant actions of ketamine. Molecular Psychiatry. April 2, 2018.
- H. Zou, et al. PDE8: A Novel Target in Airway Smooth Muscle. American Journal of Respiratory Cell and Molecular Biology. Vol. 58, No. 4. April 01 2018.
- H. Singh et al. Disruption of lipid-raft localized Gαs/tubulin complexes by antidepressants: a unique feature of HDAC6 inhibitors, SSRI and tricyclic compounds. Neuropsychopharmacology. February 2018.
- T.B. Johnstone, et al. PDE8 is Expressed in Human Airway Smooth Muscle and Selectively Regulates cAMP Signaling by β2AR-AC6. American Journal of Respiratory Cell and Molecular Biology. Dec. 2017.
- T. Togo Cell membrane disruption stimulates cAMP and Ca2+ signaling to potentiate cell membrane resealing in neighboring cells Biology Open. December 2017.
- K.A. McCrink, et al. β-Arrestin2 Improves Post-Myocardial Infarction Heart Failure via Sarco(endo)plasmic Reticulum Ca2+-ATPase-Dependent Positive Inotropy in Cardiomyocytes. Hypertension. Nov. 2017.
- Q. Wang, et al. Regulator of G protein signaling Gβ5-R7 is a crucial activator of muscarinic M3 receptor-stimulated insulin secretion. FASEB J. Jul. 7,2017.
- J. King et al. A G protein‐coupled α7 nicotinic receptor regulates signaling and TNF‐α release in microglia. FEBS. July 2017.
- J. Almaça, et al. Human beta cells produce and release serotonin to inhibit glucagon secretion from alpha cells.Cell Reports, Volume 17, Issue 12, 2016.
- P. Tewson, et al. New DAG and cAMP Sensors Optimized for Live-Cell Assays in Automated Laboratories. J Biomol Screen Dec. 11, 2015. Request Full Text PDF
- B.S. Moore, et al. Cilia have high cAMP levels that are inhibited by Sonic Hedgehog-regulated calcium dynamics. PNAS Nov. 15, 2016.
- RJ Lee, et al. Effects of Akt Activator SC79 on Human M0 Macrophage Phagocytosis and Cytokine Production. Cells. May 2024.
- R. Carey, et al. HSP90 Function is Required for T2R Bitter Taste Receptor Nitric Oxide Production and Innate Immune Responses in Human Airway Epithelial Cells and Macrophages. Cells. April 2022. (bioRxiv)
- I. Gapallawa, et al. Bitter taste receptors stimulate phagocytosis in human macrophages through calcium, nitric oxide, and cyclic-GMP signaling Cell. Mol. Life Sci. Feb, 2020 (bioRxiv)
- J. Wu, et al. Interaction Between HCN and Slack Channels Regulates mPFC Pyramidal Cell Excitability and Working Memory. bioRxiv. March 2023.
- M. Nuriya, et al. Alkyne-Tagged Dopamines as Versatile Analogue Probes for Dopaminergic System Analysis. Analytical Chemistry. July 2021.
- M. Thomas, et al. Optically activated, customizable, excitable cells. PLOS One. December 2020.
- K. Harlen, et al. Live-Cell Assays for Cell Stress Responses Reveal New Patterns of Cell Signaling Caused by Mutations in Rhodopsin, α-Synuclein and TDP-43 Frontiers Cellular Neuroscience. December 2019.
- N. Naim et al. Luminescence-activated nucleotide cyclase regulates spatial and temporal cAMP synthesis. Journal of Biological Chemistry. December 2018.
- Y.A.B. Sierra, et al. Potassium channel-based optogenetic silencing. Nature Communications. November 2018.