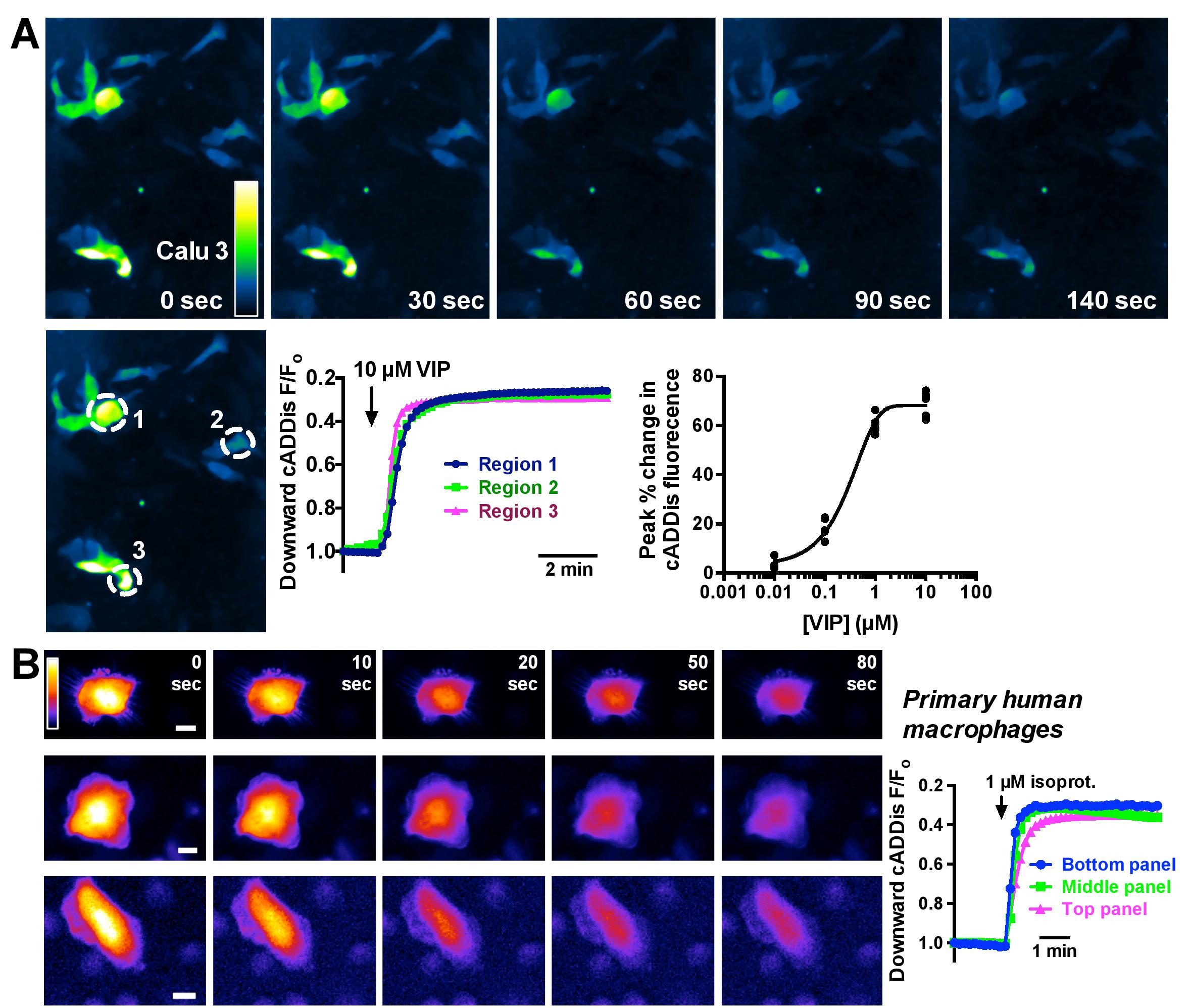
The Lee Lab at University of Pennsylvania is a diverse group of cell biologists who combine their unique scientific perspectives to the better understanding of airway disease physiology and taste perception. In other words, this lab knows the nose and is enthusiastic about the tools they use to probe the upper airway. With live cell fluorescence imaging and genetically-encoded biosensors like cADDis for cAMP and GENIE for cGMP, they study these signals in the cells that are relevant to diseases like cystic fibrosis. The Lee Lab was kind enough to share data acquired in Calu-3 bronchial cells (A) and macrophages (B ) using cADDis, so we could share it with you and other researchers who are interested in studying signaling in cell models for disease.
Rob Lee, the Principal lnvestigator of the eponymous Lee Lab, is an expert in the physiology of chronic respiratory infection and has been measuring Ca2+ and cAMP signaling for over 15 years using a variety of single-wavelength and ratiometric fluorescent protein biosensors. His critique tells it like this:
“We’ve been very happy with the downward cADDis cAMP indicator from Montana Molecular, which we’ve used in a variety of airway cell lines (16HBE, Calu-3, BEAS-2B, etc.) as well as primary human macrophages and exocrine gland acinar cells. The transfection efficiency of the BacMam is excellent in hard-to-transfect cell lines like Calu-3s (>30-40% vs <10% with lipofectamine) and we can even achieve 5-10% transduction in macrophages, which are nearly impossible to transfect with cationic lipids.
The indicator itself is brighter than many GFP-based biosensors we’ve used previously, and the dynamic range is very good. The fluorescence changes are comparable with or better than other published single-wavelength cAMP indicators (we’ve used several). We routinely observe a >60-70% change upon maximal cAMP stimulation that is reversible upon agonist washout. We’ve also been able to observe a decrease in baseline cAMP in some cells with Gi-coupled receptor stimulation. We have not been able to see that with other single-wavelength indicators we have tried. We also tried the cilia-localized ratiometric cADDis and saw nice localization and responses to isoproterenol in primary cilia. Additionally, we successfully used the cGMP indicator in several cell types, and have have found it to be better than several other cGMP indicators we’ve tried.
The ability to deliver working biosensors in BacMam has been real game-changer for us. It has allowed us to do real time imaging of cAMP/cGMP signaling in hard-to-transfect cell types that we did not previously think would possible. I’ve already recommended the cADDis to several of my colleagues and we are continuing to use the Montana Molecular products in my lab.”
Many thanks to Rob for sharing his recordings of cAMP signaling in macrophages and for his expert assessment of our assays. It’s good to know that our BacMam system can deliver to these notoriously difficult-to-transfect macrophages. And even better to know that cADDis compares so favorably to other sensors in terms of dynamic range, brightness and signal to noise.
Is your lab working in a difficult-to-transfect cell type, struggling with low signal-to-noise or photobleaching? Please email us. We’re here to help with the brightest fluorescent biosensors for live cell assays in your favorite cell type.

