Download Montana Molecular posters from SfN 2025
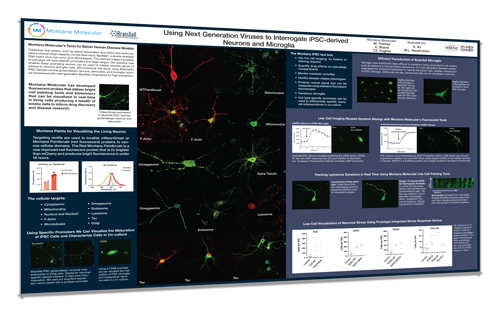
Next Generation Viruses to Interrogate iPSC-derived Neurons and Microglia
Traditional viral vectors, adeno-associated virus (AAV) and lentivirus, have a minimal cargo capacity. On the other hand, BacMam, a double stranded DNA insect virus, can carry up to 38 kilobases. This payload makes it possible to use large cell-type-specific promoters and large cargos. Our question was whether these promising vectors can be used to reliably express genes of interest in neurons and glial cells. We undertook this study using BrainXell’s iPSC-derived cortical glutamatergic neurons, astrocytes, and microglia, which we transduced with next-generation BacMan engineered for high expression. These viruses delivered genetically encoded biosensors as well as probes for mitochondria, lysosomes, omegasomes, cytoskeleton, nucleus, Golgi, and endosomes.
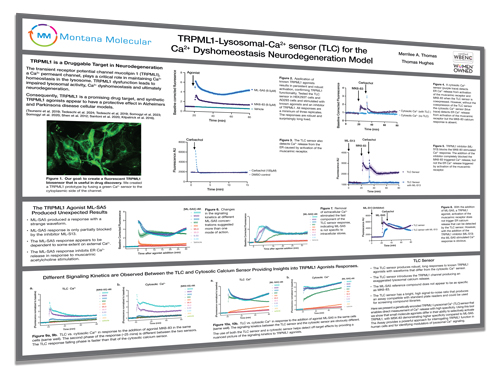
TRPML1-Lysosomal-Ca2+ sensor (TLC) for the Ca2+ Dyshomeostasis Neurodegeneration Model
Here we present a genetically encoded TRPML1 Lysosomal Ca2+ (TLC) sensor that enables direct measurement of Ca2+ release with high specificity. Using this tool we show that small molecule agonists differ in their ability to selectively activate TRPML1, with MSK-83 demonstrating higher specificity compared to ML-SA5. The Assay provides a powerful approach for interrogating TRPML1 function in human cells and for identifying modulators of lysosomal Ca2+ signaling.
Perturbed Ca2+ signaling leads to synaptic loss, amyloid and tau aggregation, mitochondrial dysfunction, lysosomal alkalinization, and superoxide production, all of which are hallmarks of neurodegenerative diseases. Evidence from Alzheimers models suggest that ER-derived Ca2+ signals disrupt mitochondria-lysosome interactions, further exacerbating cellular stress.
Schedule a discussion on TRPML1 signaling or BacMam expression with Senior Application Scientist, Scott Martinka: