Summer days: long hikes, water sports, hot dogs, fresh peaches, and plenty of sunscreen. How is it that our skin changes when exposed to sun, and why is it that those hot dogs on the grill smell so good? Two recent papers provide new glimpses of the molecular mechanisms behind appetites and suntans, revealing the functions of obscure brain nuclei, vestigial photoreceptor proteins, and fragile cellular appendages. The opsin proteins that we commonly think of—rhodopsin for example—reside on specialized sensory cilia known as photoreceptor outer segments. These receptors provide us with vision. However there are other opsins, with more obscure, non- visual, roles.1 Perhaps the most obscure is OPN3. This protein has been shown to bind retinal, but it doesn’t respond to light.2 It is widely expressed, but until recently, its function was poorly understood. A recent study from Dr. Elena Oancea’s lab at Brown University3, shows that OPN3 interacts with the MCR4 receptor to regulate receptor activity.
The MCR4 receptor is expressed by cells in the parabrachial nucleus, a brainstem region first described by C. Judson Herrick in 1905.4 Cells in this region control appetite, and MCR4 receptor knock-out mice show profound differences in appetite.5,6 The MCR4 receptor is coupled to the stimulatory Gs pathway, and activation of the receptor produces increases in cAMP. This response is beautifully illustrated in Figure 2 3, with Montana Molecular’s cADDis biosensor demonstrating the functional effect of OPN3 modulation of the MCR4 response. The OPN3 receptor appears to have a high level of constitutive activity and signals through the inhibitory pathway Gi. We can imagine a scheme whereby a constitutively active receptor would, through Gi, dampen cellular production of cAMP. However, in an interesting twist, when the authors stimulate the β1-adrenergic receptor, the OPN3 effect is gone, demonstrating that the OPN3 influence is not global, but it is instead restricted to modulating MCR4. This may be due to subcellular cAMP signaling, 7 where receptors and enzymes are clustered together as functionally independent “signalsomes.”
MCR4 is thought to function in the primary sensory cilium of the cell.8 Cilia are remarkably thin, small cellular appendages that we now know are present on many cells.9 Could it be possible that appetite is controlled by the interactions of OPN3 and MCR4 in this remarkably small cellular appendage protruding from certain neurons of the parabrachial nucleus? This would be true if they coupled to ion channels because remarkably little current in a compact structure is sufficient to produce important cellular change. Indeed, there are fascinating reports of MCR4 coupling to the Kir7.1 ion channel,10 and in this recent report OPN3 couples to the channel as well.3 It remains to be seen whether Kir7.1 actually localizes to the cilium.
OPN3 interacts with the related MCR1 receptor to counter its actions much like it does with MCR4. MCR1 is also on the cilia, but on the cilia of melanocytes, the cells in our skin that produce melanin to protect us from UV light. Much as it does with MCR4, the OPN3 appears to negatively regulate the agonist-induced production of cAMP.2 Recently, a team from Shandong Normal University in Jinan China11 used Montana Molecular’s cilia targeted cADDis biosensor12 to demonstrate that the MCR1 receptor produces long-lived, localized cAMP responses in the cilia of these cells. Beyond simply being trafficked and localized to the cilium, Tian and colleagues demonstrate that stimulation of the receptor appears to drive the production of a cilium in culture, which is a very provocative finding and offers a pharmacological approach towards controlling cilia growth.
Like the best summertime reads, these papers are well written, nicely illustrated, and have fascinating plot twists that you don’t expect.
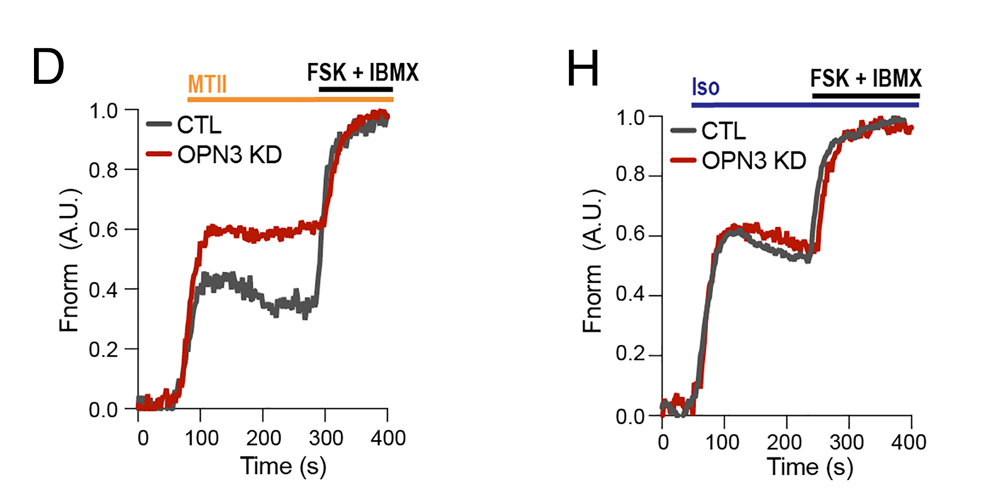
Fig 2 from Hypothalamic opsin 3 suppresses MC4R signaling and potentiates Kir7.1 to promote food consumption.
In this experiment the authors use the cADDis biosensor to follow cAMP levels in the immortalized hypothalamic neuronal cell line GT1-7. Panel D illustrates how Melotan II (MTII) stimulates the MCR4 receptor to increase cytosolic cAMP. The total cADDis sensor range is measured at the end of the experiment by adding forskolin and IBMX (FSK +IBMX). In cells with reduced expression of the OPN3 receptor, this response is significantly larger than in control cells (CTL) which indicates that OPN3 reduces the MCR4 mediated production of cAMP. This reduction does not, however, appear to be global, because the experiment in panel H shows that stimulation of the β1 adrenergic receptor with isoproterenol (Iso) is not suppressed by OPN3
- Andrabi, M., Upton, B. A., Lang, R. A. & Vemaraju, S. An expanding role for nonvisual opsins in extraocular light sensing physiology. Annu. Rev. Vis. Sci. 9, 245–267 (2023).
- Ozdeslik, R. N., Olinski, L. E., Trieu, M. M., Oprian, D. D. & Oancea, E. Human nonvisual opsin 3 regulates pigmentation of epidermal melanocytes through functional interaction with melanocortin 1 receptor. Proc. Natl. Acad. Sci. U. S. A. 116, 11508–11517 (2019).
- Haddad, H. K. et al. Hypothalamic opsin 3 suppresses MC4R signaling and potentiates Kir7.1 to promote food consumption. Proc. Natl. Acad. Sci. U.S.A. 122, (2025).
- Herrick, C. J. The central gustatory paths in the brains of bony fishes. Studies from the Neurological Laboratory of Denison University. No. XVIII. J. Comp. Neurol. Psychol. 15, 375–456 (1905).
- Huszar, D. et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88, 131–141 (1997).
- Garfield, A. S. et al. A neural basis for melanocortin-4 receptor-regulated appetite. Nat. Neurosci. 18, 863–871 (2015).
- Scott, J. D., Dessauer, C. W. & Taskén, K. Creating order from chaos: cellular regulation by kinase anchoring. Annu. Rev. Pharmacol. Toxicol. 53, 187–210 (2013).
- Bernard, A. et al. MRAP2 regulates energy homeostasis by promoting primary cilia localization of MC4R. JCI Insight 8, (2023).
- Smith, E. F. & Rohatgi, R. Cilia 2010: the surprise organelle of the decade. in Science signaling vol. 4 mr1–mr1 (AAAS, 2011).
- Ghamari-Langroudi, M. et al. G-protein-independent coupling of MC4R to Kir7.1 in hypothalamic neurons. Nature 520, 94–98 (2015).
- Tian, X. et al. Melanocortin 1 receptor mediates melanin production by interacting with the BBSome in primary cilia. PLoS Biol. 22, e3002940 (2024).
- Moore, B. S. et al. Cilia have high cAMP levels that are inhibited by Sonic Hedgehog-regulated calcium dynamics. Proceedings of the National Academy of Sciences 113, 13069–13074 (2016).
