BacMam-packaged human RAMP1, RAMP2, and RAMP3 are now available from Montana Molecular!
Receptor Activity-Modifying Proteins (RAMPs) can regulate the function and specificity of G-protein-coupled receptors – influencing their trafficking, ligand selectivity, and signaling. BacMam viruses are easily co-transduced, allowing RAMPs to be expressed alongside Montana Molecular’s suite of GPCR expression vectors and signaling assays for Arrestin, cAMP, DAG, and Calcium.
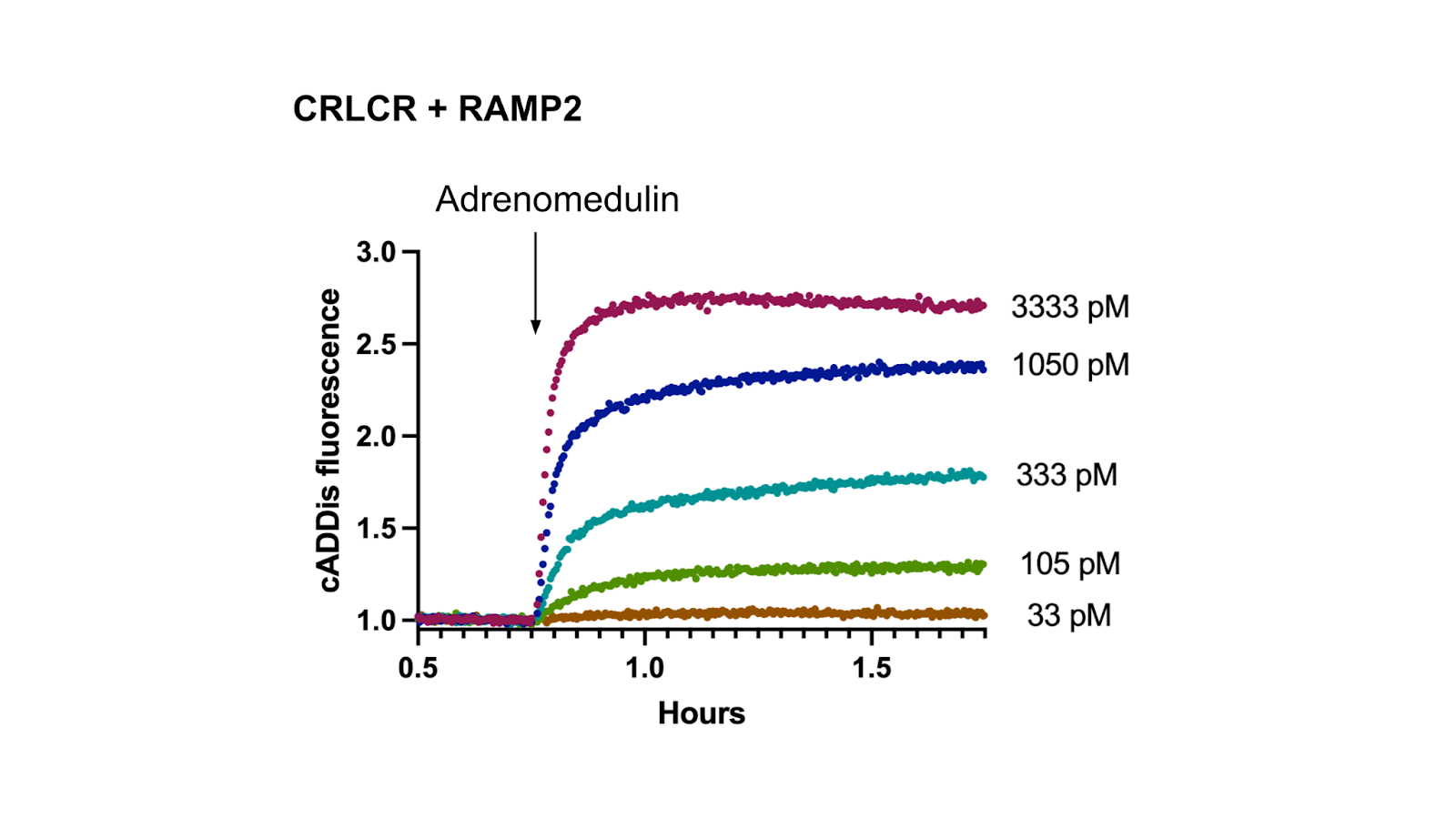
The figure below shows some example data gathered in HEK293T cells co-expressing the cADDis cAMP sensor, Calcitonin Receptor-like Receptor (also known as CRLCR, CALCRL, or CRLR), and RAMP2.
Figure 1: cAMP signaling after Adrenomedulin addition in HEK293T cells expressing cADDis, CRLR, and RAMP2.
RAMPs were first discovered through an expression cloning strategy. McLatchie and colleagues were searching for a cDNA that would dramatically increase the calcitonin-gene related peptide (CGRP) response in xenopus oocytes. (McLatchie et al. 1998). They found a relatively small gene product, which was not itself a receptor. Instead it somehow increased the surface expression of a GPCR as though it was a chaperone. Surprisingly, it does more than simply modify protein trafficking.
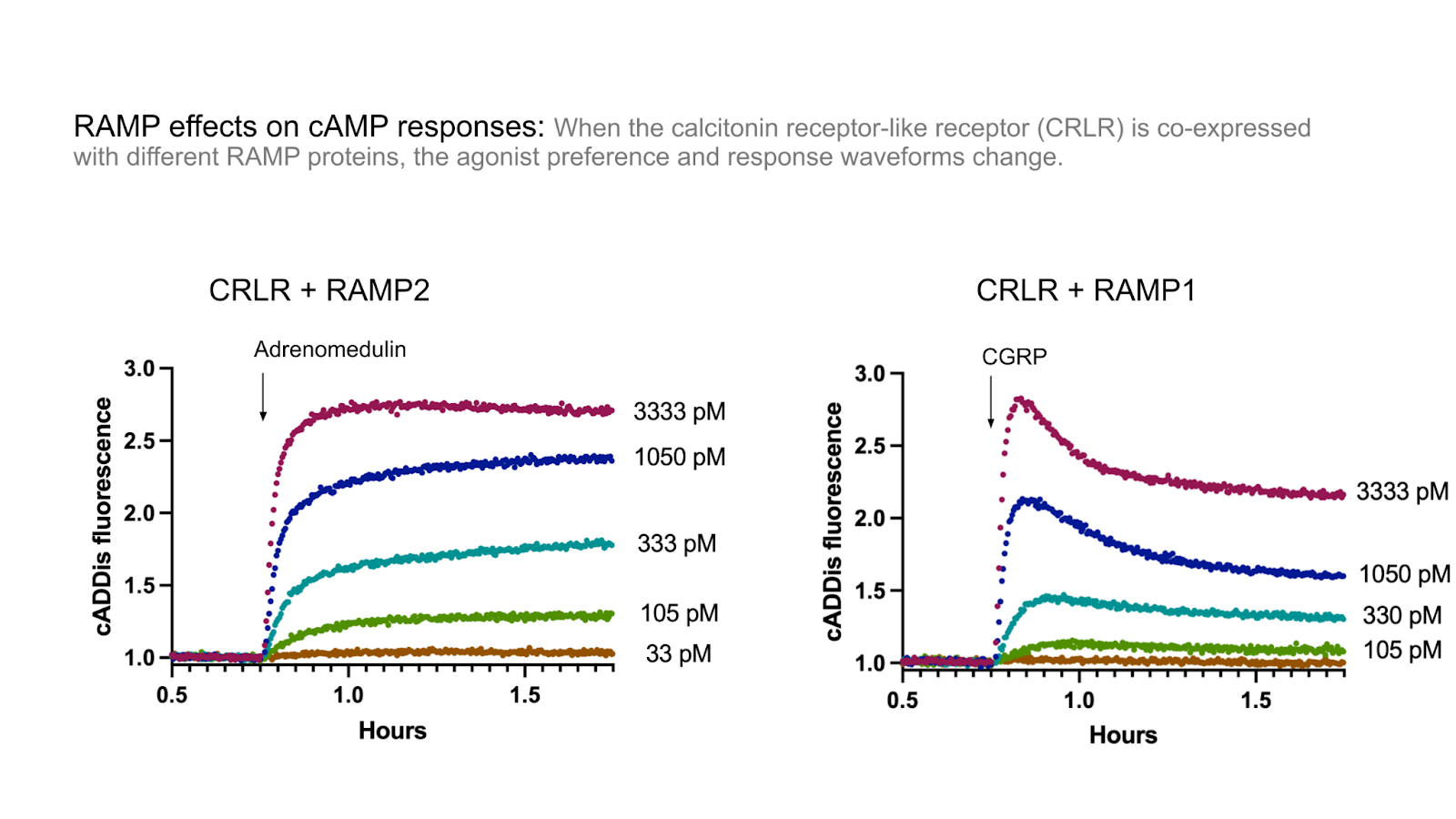
When the calcitonin receptor-like receptor (CRLR) was co-expressed with RAMP1, it formed the calcitonin-gene-related peptide receptor (CGRPR), with a high-affinity for CGRP. However when it was expressed with the related RAMP2 or RAMP3 proteins, it formed the adrenomedullin I receptor (AM1R) and the adrenomedullin II receptor (AM2R), respectively, with a lower affinity for CGRP. (McLatchie et al. 1998) (Kotliar et al. 2023).
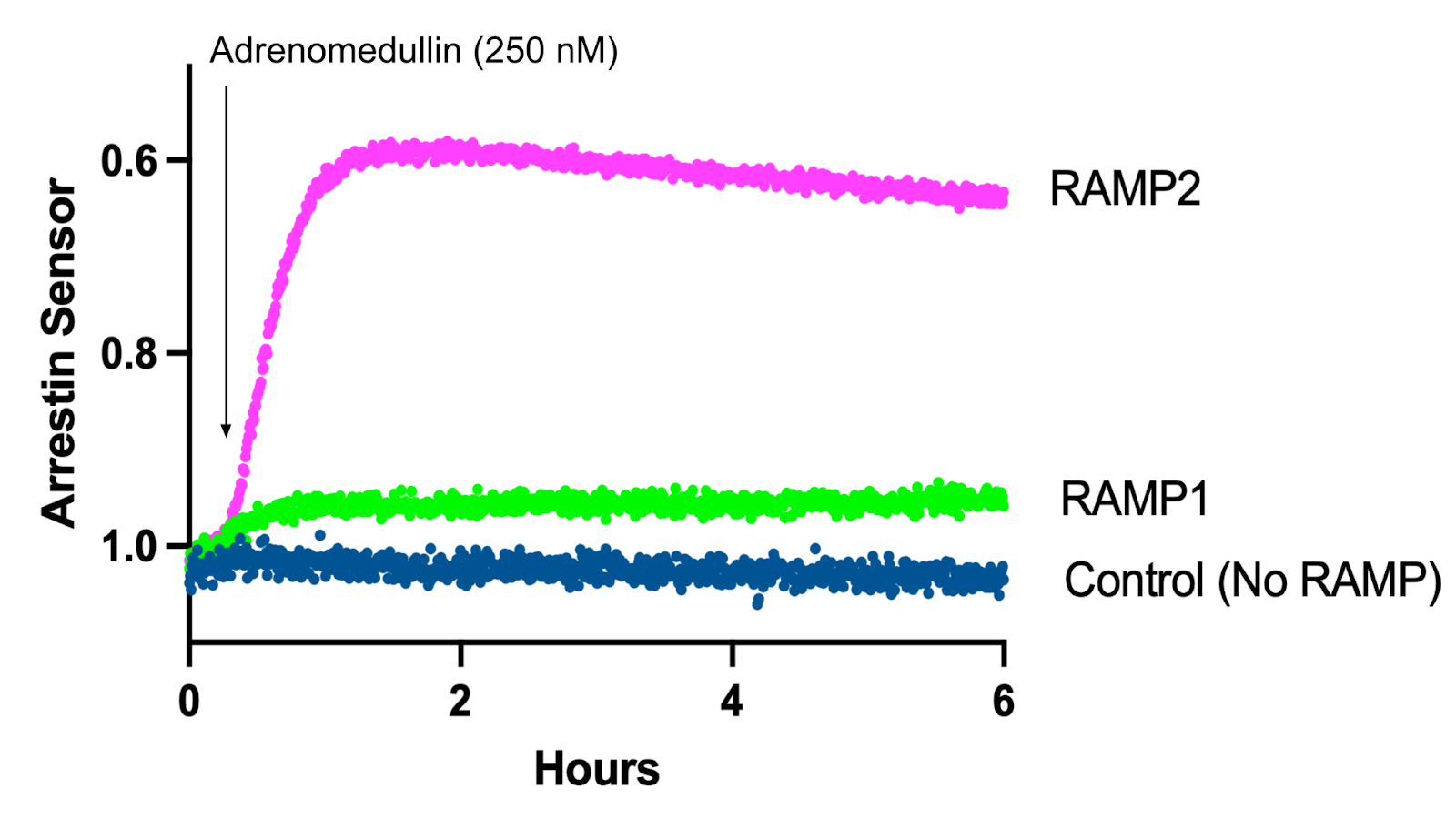
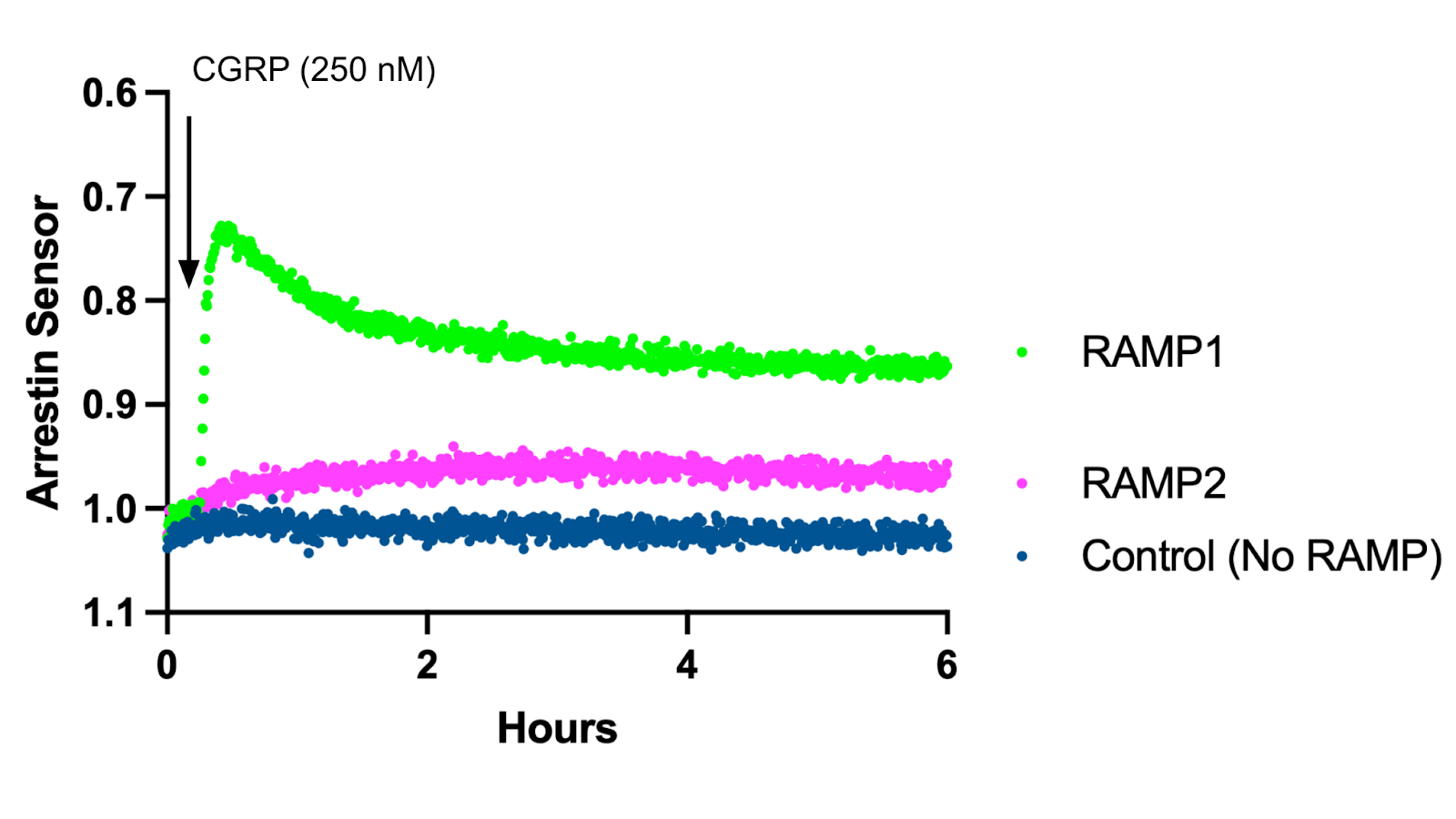
Using our beta-arrestin 2 recruitment biosensor, Borealis, we’ve explored the role RAMPs play in changing arrestin recruitment through the CRLR receptor. Indeed, Figure 2 shows adrenomedullin robustly recruits arrestin in cells co-expressing RAMP2 and CRLR, but fails to trigger recruitment in cells expressing RAMP1 and CRLR. Conversely, in Figure 3 addition of CGRP led to arrestin recruitment in cells expressing RAMP1 and CRLR, but not RAMP2 and CRLR.
Figure 2: Arrestin Recruitment after Adrenomedullin addition in HEK293T cells expressing Borealis, CRLR, and either RAMP1, RAMP2, or no RAMP.
Figure 3: cAMP signaling after CGRP addition in HEK293T cells expressing cADDis, CRLR, and either RAMP1, RAMP2.
Figure 3: Arrestin Recruitment after CGRP addition in HEK293T cells expressing Borealis, CRLR, and either RAMP1, RAMP2, or no RAMP.
There is recent, growing evidence for RAMPs associating with GPCRs other than CRLR. An intriguing bioinformatics study indicates that the RAMPs have co-evolved with a variety of different GPCRs (Barbash et al. 2017). More definitive biochemical experiments have shown that 122 out of 215 screened GPCRs interact with at least one of the three RAMP proteins; and 23 GPCRs form complexes with RAMPs when natively expressed (Kotliar et al. 2023). Montana Molecular has a growing catalog of GPCRs packaged in BacMam, ready to be co-transduced with RAMPs by researchers interested in exploring these interactions.
References
Barbash, S., Lorenzen, E., Persson, T., Huber, T., & Sakmar, T. P. (2017). GPCRs globally coevolved with receptor activity-modifying proteins, ramps. Proceedings of the National Academy of Sciences, 114(45), 12015–12020. https://doi.org/10.1073/pnas.1713074114
Kotliar, I. B., Bendes, A., Dahl, L., Chen, Y., Saarinen, M., Ceraudo, E., Dodig-Crnković, T., Uhlén, M., Svenningsson, P., Schwenk, J. M., & Sakmar, T. P. (2023). Expanding the GPCR-ramp interactome. bioRxiv. https://doi.org/10.1101/2023.11.22.568247
Kotliar, I. B., Lorenzen, E., Schwenk, J. M., Hay, D. L., & Sakmar, T. P. (2022). Elucidating the interactome of G protein-coupled receptors and receptor activity-modifying proteins. Pharmacological Reviews, 75(1), 1–34. https://doi.org/10.1124/pharmrev.120.000180
McLatchie, L. M., Fraser, N. J., Main, M. J., Wise, A., Brown, J., Thompson, N., Solari, R., Lee, M. G., & Foord, S. M. (1998). Ramps regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature, 393(6683), 333–339. https://doi.org/10.1038/30666
BacMam Gene Delivery
Custom BacMam preparations to deliver your gene of interest to mammalian cells.