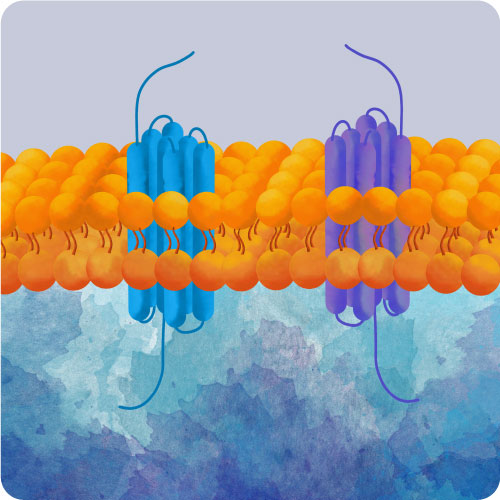
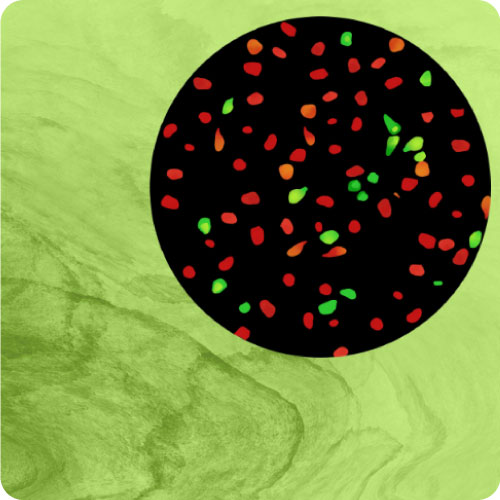
GPCR Biology
G-Protein Coupled Receptors (GPCRs) are one of the largest receptor families in the genome and are essential for the healthy function of nearly every organ in the body. GPCRs are also important targets for therapeutic drugs. Increase your understanding of drug effects and GPCR biology with bright fluorescent assays in living cells.
With our GPCR Assays in Living Cells, you can:
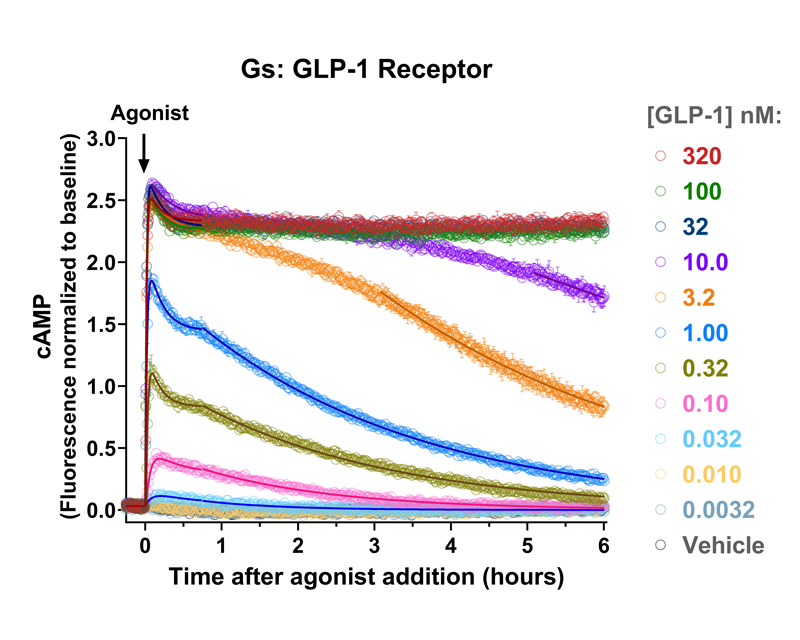
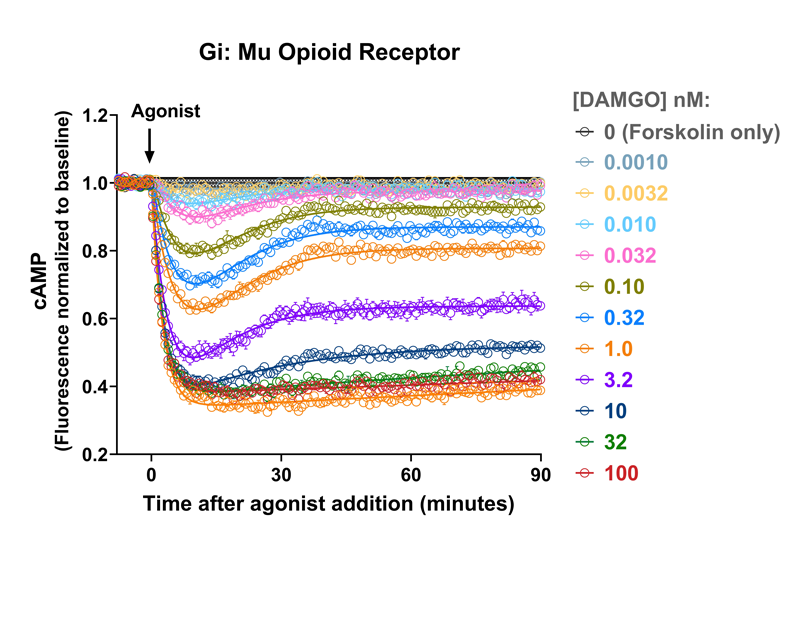
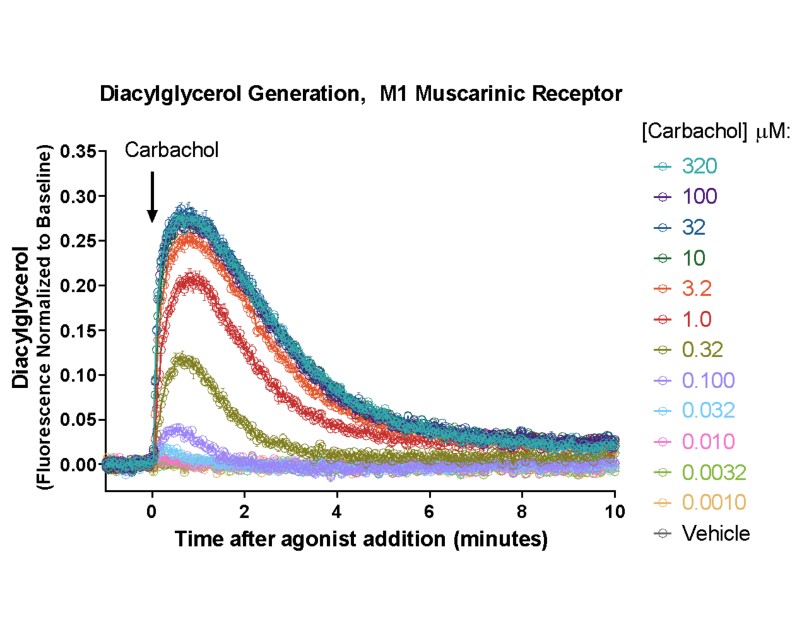
- Detect kinetic Gs, Gi, and Gq mediated responses in living cells
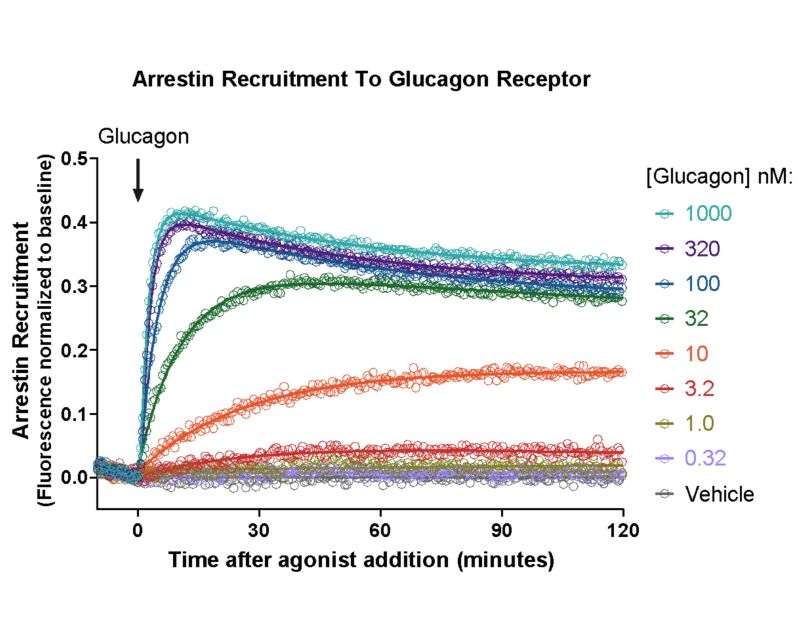
- Measure arrestin recruitment
- Combine multiple assays in the same cell population
- Express sensors and run assays in disease relevant cell types
- Detect fluorescence on imaging systems or automated plate readers (Z’ > 0.8)
- Quantify agonist bias
- De-orphanize receptors
Detecting Arrestin
Fluorescent Borealis arrestin assays to detect arrestin recruitment at specific GPCRs.
Measuring GPCR Signaling Kinetics
See the Assay Guidance Manual chapter from NIH NCATS:
Express GPCRs, GRKs, and RAMPs with BacMam
Montana Molecular offers a growing suite of RAMPs, GRKs, and GPCRs packaged in a modified Baculovirus vector, BacMam. BacMam transduction is a low-cost, effective method to express GPCRs in your cells of interest, including in primary and iPSC derived cells. See low cell-to-cell variability in expression, get consistent results from experiment to experiment, and titrate expression to your desired level.
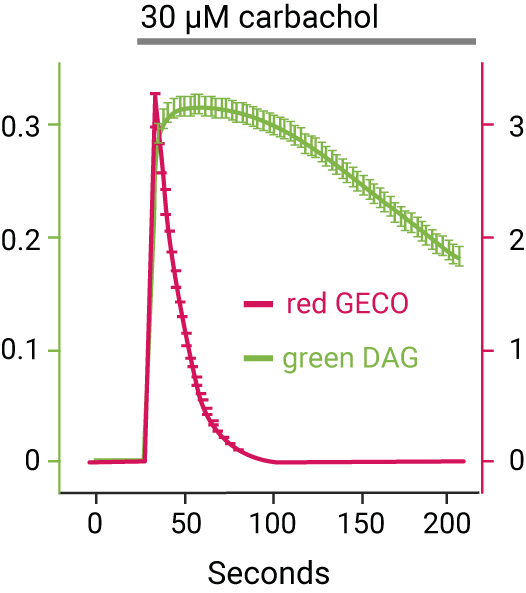
Continuous Multiplex Measurements
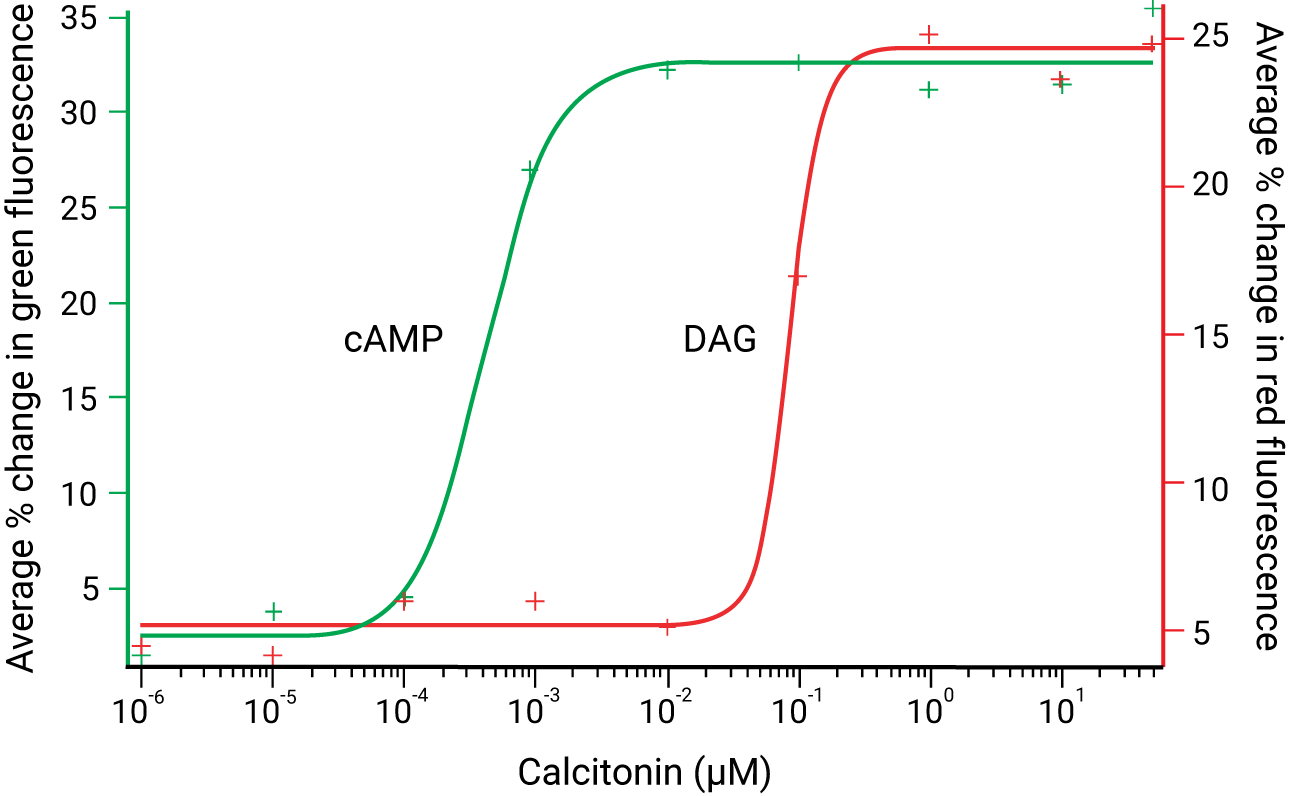
Red fluorescent DAG sensor multiplexed with cADDis, a green fluorescent cAMP sensor, indicates Gs & Gq signaling via a calcitonin receptor.
Assays in Primary Cultures or iPSCs
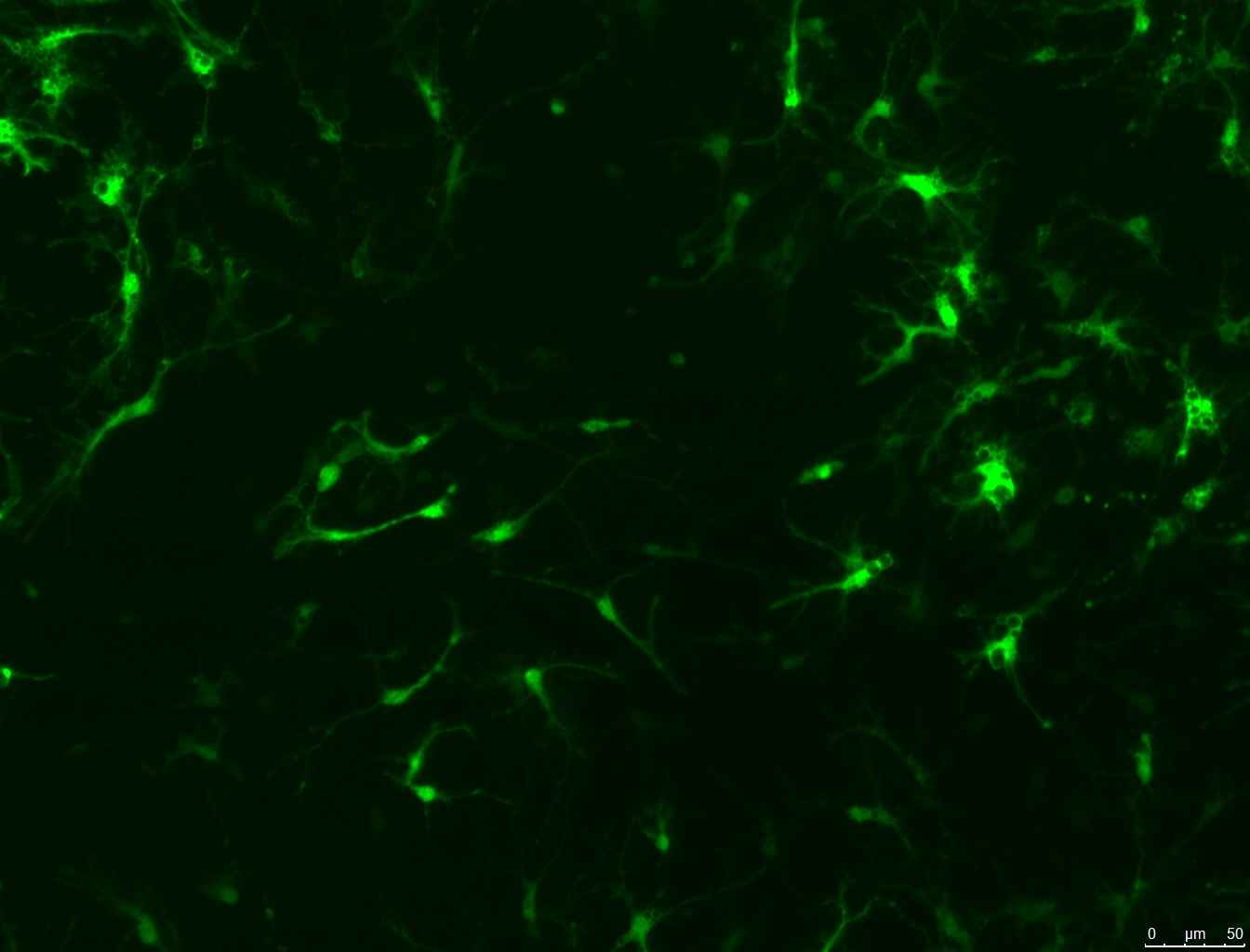
Detect GPCR mediated responses in cells relevant to your disease, drug target, or biology of interest. Our BacMam-packaged sensors have been used in neurons, cardiomyoctes, islets, and many more primary and iPSC derived cells. Examples can be found on our Scientific Publications page. Alternate promoters or viral vectors are available by request. Pictured, at right, are the cADDis cAMP Assay in primary striatal neurons and the Red GECO calcium assay in nCardia’s Cor.4u cardiomyocytes.
Simple Protocol
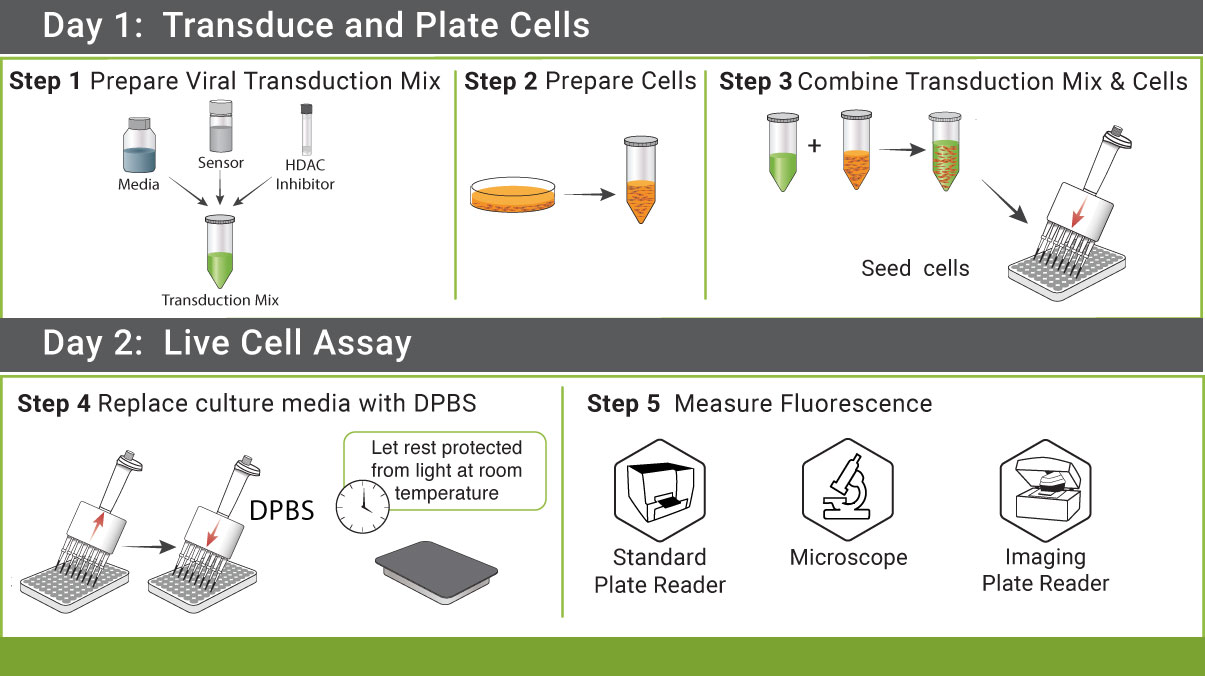
We strive to create simple protocols with minimal liquid handling. Cell lysis, IBMX, enzymes, and co-factors are not necessary for these assays. Add our sensors to your cells, incubate, add drug, and measure fluorescent changes.
Read more on each of our specific GPCR Assays and receptors:
Shop Other Assay Kits by Category
Recent Publications
GPCR Assay References
Borealis Arrestin Assay References
- Y. Manchada, et al. Glucagon-like peptide-1 receptor C-terminal tail phosphorylation determines signalling responses from pancreatic β-cells. bioRxiv. November 2025.
- A. Ippolito, et al. Evidence that 5-HT2A receptor signalling efficacy and not biased agonism differentiates serotonergic psychedelic from non-psychedelic drugs. British Journal of Pharmacology. June 2025.
- E. Billard, et al. Pharmacological characterization of cannabidiol as a negative allosteric modulator of the 5-HT2A receptor. Cellular Signaling. January 2025.
- I. Davies, et al. Chronic GIPR agonism results in pancreatic islet GIPR functional desensitisation. Molecular Metabolism. January 2025.
- A. Ippolito, et al. Increased 5-HT2A receptor signalling efficacy differentiates serotonergic psychedelics from non-psychedelics. bioRxiv. June 2024.
- H. Schiff, et al. β-arrestin-biased proteinase-activated receptor-2 antagonist C781 limits allergen-induced airway hyperresponsiveness and inflammation. British Journal of Pharmacology. June 2022.
cADDis cAMP Assay References
- G. Austin, et al. GLP-1R associates with VAPB and SPHKAP at ERMCSs to regulate β-cell mitochondrial remodelling and function. Nature Communications. December 2025.
- S. Chen, et al. Spatially diffuse cAMP signalling with oppositely biased GLP-1 receptor agonists in β-cells despite differences in receptor localisation. Molecular Metabolism. December 2025.
- S. Orfanos, et al. The differential effects of cAMP mobilizing agents on TGF-β-induced extracellular matrix in human lung-derived fibroblasts: insights into therapeutic targets for lung fibrosis. Respiratory Research. November 2025.
- M. Dickerson, et al. Glycolytic activation of β-cell Na+/K+-ATPases containing β1-subunits accelerates Na+ extrusion, prolonging the duration of Ca2+ oscillations but decreasing insulin secretion. Molecular Metabolism. December 2025.
- Y. Manchada, et al. Glucagon-like peptide-1 receptor C-terminal tail phosphorylation determines signalling responses from pancreatic β-cells. bioRxiv. November 2025.
- C. Metrick, et al. GPR17 structure and agonism with small molecules and oxysterols. bioRxiv. November 2025.
- S. Zervou, et al. Effect of whole-body creatine-deficiency on secretion of insulin and glucagon from isolated mouse islets. SSRN. October 2025.
- Mark Lee & Keren Hilgendorf. Prostaglandin E2 inhibits adipogenesis through the cilia-dependent activation of ROCK2. Journal of Cell Science. September 2025.
- R. Nassini, et al. Targeting prostaglandin E2 receptor 2 in Schwann cells inhibits inflammatory pain but not inflammation. Nature Communications. September 2025.
- Tatsuru Togo. Disruption of the cell membrane triggers Ca2+ mobilization using P2Y receptors and subsequent cAMP synthesis in cells surrounding wounded MDCK cells. Biochemical and Biophysical Research Communications. September 2025.
- C. Sturaro, et al. Activation of peripheral NOP receptors reduces periorbital mechanical allodynia evoked by CGRP in mice. British Journal of Pharmacology. August 2025.
- T. Cacciottolo, et al. Glucagon Receptor Deficiency Causes Early-Onset Hepatic Steatosis. Diabetes. August 2025.
- J. Chadwick, et al. Dietary-dependent sensitization of neuronal leptin signaling promotes neural repair after injury via cAMP and gene transcription. Neuron. August 2025.
- K. Berger, et al. Design of a peripherally biased NPSR1 antagonist for neuropeptide S induced inflammation. Bioorganic & Medicinal Chemistry Letters. August 2025.
- C. Gao, et al. Semaglutide drives weight loss through cAMP-dependent mechanisms in GLP1R-expressing hindbrain neurons. bioRxiv. August 2025.
- Y. Manchanda, et al. Glycaemic and bodyweight effects of GIPR coding variation reflect differences in both surface expression and intrinsic functional impairment. medRxiv. August 2025.
- S. Orfanos, et al. The differential effects of cAMP mobilizing agents on TGF-β-induced extracellular matrix in human lung-derived fibroblasts: Insights into therapeutic targets for lung fibrosis. Research Square. August 2025.
- N. Saito, et al. Activation of G protein-coupled parathyroid hormone receptors in rat incisor odontoblasts promotes mineralization via cyclic adenosine monophosphate, not Ca2+ signalling: In vitro study. International Endodontic Journal. July 2025.
- M. Merz, et al. Downstream Signaling of Muscarinic M4 Receptors Is Regulated by Receptor Density and Cellular Environment. Pharmacology Research & Perspectives. May 2025.
- B. Wysolmerski, et al. Conformational biosensors delineate endosomal G protein regulation by GPCRs. bioRxiv. May 2025.
- Paul G. DeCaen & Louise F. Kimura. Methods to Assess Neuronal Primary Cilia Electrochemical Signaling. Journal of Cellular Physiology. April 2025.
- A. Oqua, et al. Molecular mapping and functional validation of GLP-1R cholesterol binding sites in pancreatic beta cells. eLife. April 2025. (bioRxiv)
- L. Coassolo, et al. Prohormone cleavage prediction uncovers a non-incretin anti-obesity peptide. Nature. March 2025.
- R. Schuck, et al. Cholesterol inhibits assembly and oncogenic activation of the EphA2 receptor. Nature Communications Biology. March 2025. (bioRxiv)
- H. Haddad, et al. Hypothalamic opsin 3 suppresses MC4R signaling and potentiates Kir7.1 to promote food consumption. PNAS. February 2025.
- E. Blythe, et al. Endocytosis sculpts distinct cAMP signal transduction by endogenously coexpressed GPCRs. bioRxiv preprint. February 2025.
- S. Orfanos, et al. The differential effects of cAMP mobilizing agents on inhibition of TGF-β-induced extracellular matrix and growth factor expression in human lung fibroblasts. Authorea. February 2025.
- G. Austin, et al. GLP-1R associates with VAPB and SPHKAP at ERMCSs to regulate β-cell mitochondrial remodelling and function. bioRxiv. February 2025.
- A. Klein, et al. Inhibition of adenylyl cyclase 1 (AC1) and exchange protein directly activated by cAMP (EPAC) restores ATP-sensitive potassium (KATP) channel activity after chronic opioid exposure. bioRxiv. February 2025.
- I. Davies, et al. Chronic GIPR agonism results in pancreatic islet GIPR functional desensitisation. Molecular Metabolism. January 2025.
DAG Assay References
- E. Lukovic, et al. Design and Evaluation of Novel Ginger 6-Shogaol-Inspired Phospholipase C Inhibitors to Enhance β-Agonist-Induced Relaxation in Human Airway Smooth Muscle. Journal of Medicinal Chemistry. June 2025.
- E. Billard, et al. Pharmacological characterization of cannabidiol as a negative allosteric modulator of the 5-HT2A receptor. Cellular Signaling. January 2025.
- X. Chen, et al. Roles for PKC signaling in chromaffin cell exocytosis. Biophysical Journal. December 2024.
- A. Hamilton, et al. Nicotinic signaling stimulates glucagon secretion in mouse and human pancreatic α-cells. Diabetes. October 2024.
- X. Chen, et al. A PACAP-activated network for secretion requires coordination of Ca2+ influx and Ca2+ mobilization. Molecular Biology of the Cell. May 2024. (bioRxiv)
- S. Datta, et al. APOL1-mediated monovalent cation transport contributes to APOL1-mediated podocytopathy in kidney disease. The Journal of Clinical Investigation. January 2024. (Supplemental Data)
- Z. Miller, et al. Lidocaine induces apoptosis in head and neck squamous cell carcinoma through activation of bitter taste receptor T2R14. Cell Reports. November 2023. (bioRxiv)
- N. Zaïmia, et al. GLP-1 and GIP receptors signal through distinct β-arrestin 2-dependent pathways to regulate pancreatic β cell function. Cell Reports. October 2023.
- G. Sanchez, et al. Coincident Regulation of PLCβ Signaling by Gq-Coupled and μ-Opioid Receptors Opposes Opioid-Mediated Antinociception. Molecular Pharmacology. December 2022.
- Z. Miller, et al. Lidocaine Induces Apoptosis in Head and Neck Squamous Cell Carcinoma Cells Through Activation of Bitter Taste Receptor T2R14. bioRxiv. November 2022.
- M. Doepner, et al. Endogenous DOPA inhibits melanoma through suppression of CHRM1 signaling. Science Advances. September 2022.
PIP2 Assay References
- V. Chandar, et al. Intercellular contact is sufficient to drive Fibroblast to Myofibroblast transitions. arXiv. March 2025.
- L. Brueggemann, et al. Structural Determinants of Kv7.5 Potassium Channels that Confer Changes in Phosphatidylinositol 4,5-Biphosphate (PIP2) Affinity and Signaling Sensitivity in Smooth Muscle Cells. Molecular Pharmacology. March 2020.
- L.Liu, et al. Gαq sensitizes TRPM8 to inhibition by PI(4,5)P2 depletion upon receptor activation. J.Neurosci. May 2019.
- , and Teaching an Old Drug New Tricks: Agonism, Antagonism, and Biased Signaling of Pilocarpine through M3 Muscarinic Acetylcholine Receptor Molecular Pharmacology Nov. 2018
GECO Calcium Assay References
- X. Chen, et al. Roles for PKC signaling in chromaffin cell exocytosis. Biophysical Journal. December 2024.
- X. Chen, et al. In vitro and in vivo inhibition of the host TRPC4 channel attenuates Zika virus infection. EMBO Molecular Medicine. July 2024.
- X. Chen, et al. A PACAP-activated network for secretion requires coordination of Ca2+ influx and Ca2+ mobilization. Molecular Biology of the Cell. May 2024. (bioRxiv)
- C. Amos, et al. Membrane lipids couple synaptotagmin to SNARE-mediated granule fusion in insulin-secreting cells. Molecular Biology of the Cell. December 2023.
- J. Wu, et al. Interaction Between HCN and Slack Channels Regulates mPFC Pyramidal Cell Excitability and Working Memory. bioRxiv. March 2023.
- M. Thomas, et al. Optically activated, customizable, excitable cells. PLOS One. December 2020.
- L. Liu, et al. Diacylglycerol kinases regulate TRPV1 channel activity. Journal of Biological Chemistry. April 2020.
- S. Hoare, et al. A kinetic method for measuring agonist efficacy and ligand bias using high resolution biosensors and a kinetic data analysis framework. Nature Scientific Reports Feb 2020.
- K. Harlen, et al. Live-Cell Assays for Cell Stress Responses Reveal New Patterns of Cell Signaling Caused by Mutations in Rhodopsin, α-Synuclein and TDP-43 Front. Cell. Neurosci.,December 2019