In a recent paper, Peng and colleagues (Peng et al. 2023) use a variety of new approaches, and fresh ideas, to create a molecular model for how the cells of the carotid body sense the oxygen levels in our blood and use this to control our breathing. Surprisingly, key clues to solving this mystery come from bad smells and the olfactory system.
Cellular Oxygen Detection
Every medical student learns about the carotid body, a small organ, perched at the bifurcation of the common carotid artery. There, the cells of the body detect changes in the levels of circulating oxygen, and in turn pass messages to the respiratory center through the glossopharyngeal nerve. It is difficult to imagine a more important reflex than the one that controls the rate of breathing, yet we still have a great deal to learn about how these cells actually detect oxygen levels. What are the actual receptors? What second messengers are involved, and how does this signaling change transmitter release?
The sensory cells of the carotid body express a peculiar enzyme cystathionine-γ-lyase that generates hydrogen sulfide (H2S), a colorless gas with the odor of rotten eggs. This sulfurous gas leads to the persulfidation of the amino acid cysteine. While it has been established that low levels of oxygen lead to the production of H2S, the mystery of which proteins, and which cysteines, were the target of the H2S remained.
Ying-Jie Peng and his colleagues at University of Chicago began to unravel this mystery when they realized that the carotid body in mice expresses olfactory receptor 78, also known as Olfr78, Olr59 in rats and OR51E2 in humans. What better protein could there be to detect rotten eggs than an olfactory receptor? Would persulfidation of this G protein-coupled receptor trigger signaling?
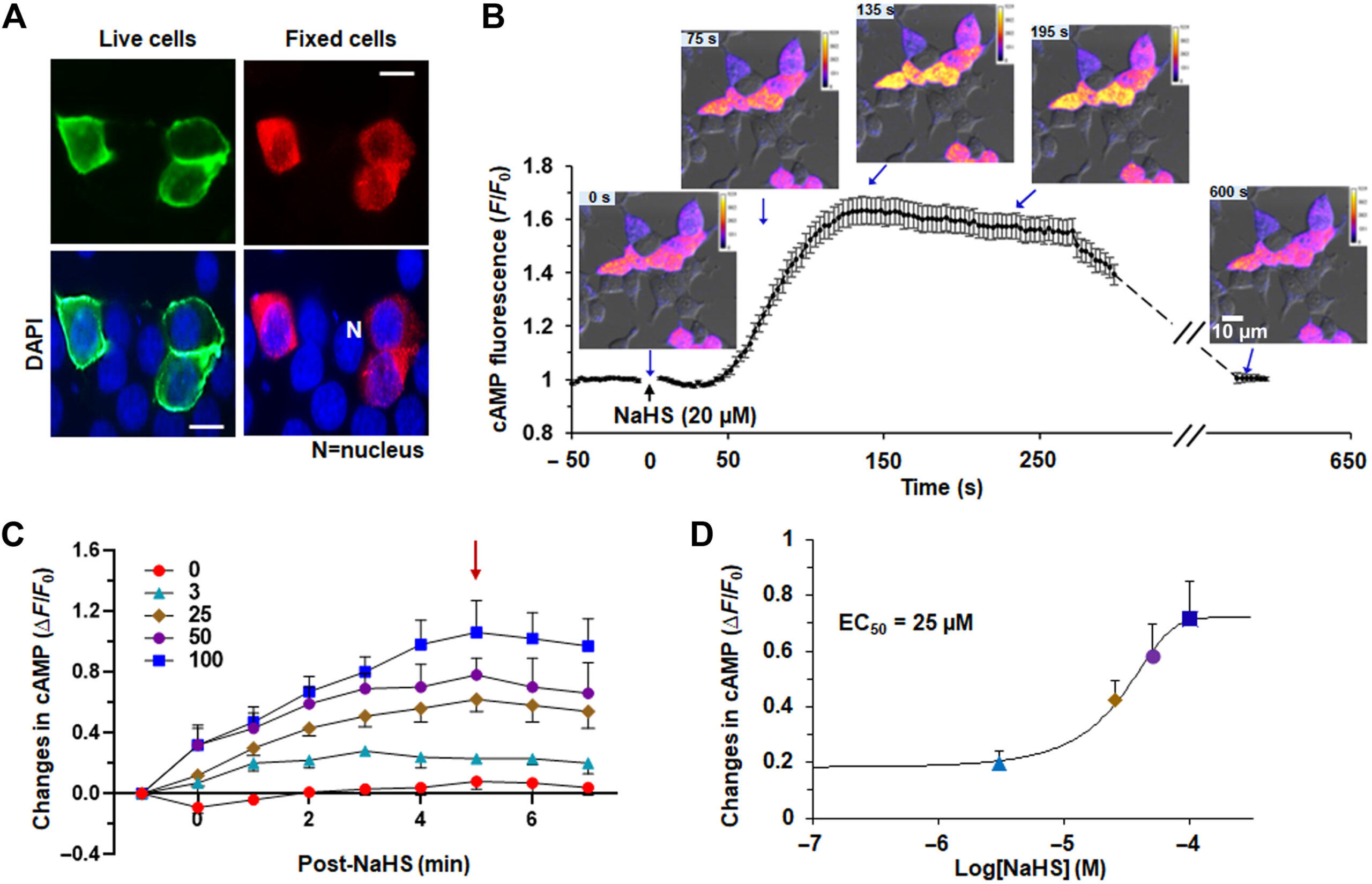
Figure one from Peng et al. 2023, demonstrates that the olfactory receptor 78 traffics nicely to the cell surface in live cells (A), and that NaHS can activate the receptor, producing an increase in intracellular cAMP (B). This increase was also detectable on a plate reader, so they were able to collect the dose response function for NaHS.
Olfactory receptor signaling
Testing this idea is experimentally challenging: many olfactory receptors fail to traffic to the cell surface in standard HEK 293 cells. Worse H2S is a short-lived molecule so slow end-point assays for cAMP would be poorly suited to detecting the relevant signaling. The authors added an epitope tag to the olfactory receptor 78 and discovered that this particular receptor does traffic to the cell surface in a heterologous system. Encouraged, the authors then expressed both the receptor and Montana Molecular’s cADDis biosensor for cAMP, and clearly demonstrated that H2S activates the receptor through persulfidation of cysteine 240 of the receptor protein. Since the cADDis sensor fluorescence is bright enough to read on automated plate readers, the authors collected the dose response properties of the response and the EC50 values are 25 uM, which is considerably more sensitive than the reference compound acetate (millimolar efficacy).
Once the persulfidation and activation of olfactory receptor 78 was established, the remaining parts of the puzzle fell into place: receptor activation raises the activity of adenylyl cyclase 3, much as it does in an olfactory cell. The resulting increase in cAMP causes the opening of cyclic nucleotide-gated ion channels, and in turn, the depolarized cell increases transmitter release. The authors sagely point out that there are still important details to be uncovered, but their contribution is clear: a powerful new model for understanding and manipulating the signaling pathways that enable us to breathe is a significant advancement for the study of hypoxia and its role in disease.
Ying-Jie Peng et al. Hypoxia sensing requires H2S-dependent persulfidation of olfactory receptor 78. Sci. Adv. 9, eadf3026 (2023). DOI:10.1126/sciadv.adf3026