Form and Function
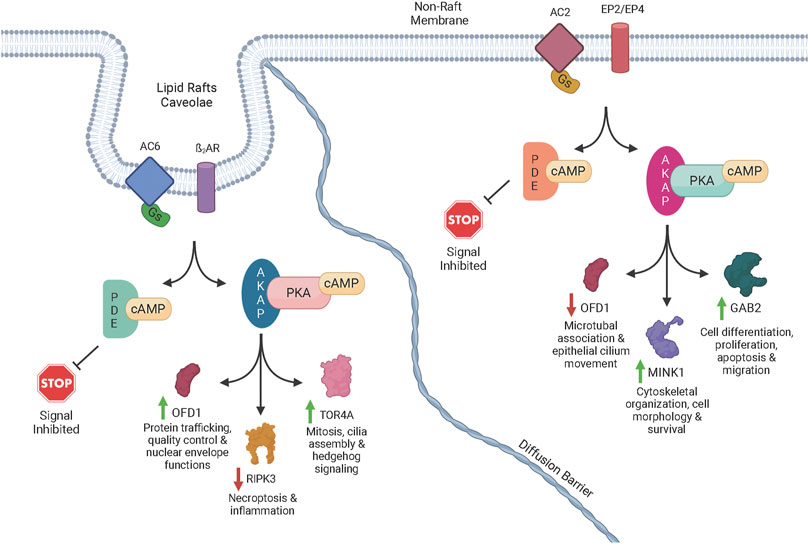
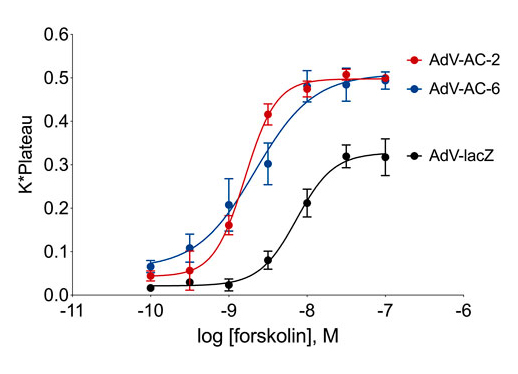
Consequences of subcellular cAMP signaling revealedIn a recent paper, Isabella Cattani-Cavalieri and her colleagues in Ren Ostrom’s laboratory found a new, powerful way to examine the functional consequences of different cAMP signalsomes (Cattani-Cavalieri et al., 2023). There has long been a suspicion that there are small, discrete cAMP signaling domains within the cell. These are often referred to as “signalsomes”, and some believe that these different micro-signaling domains explain how different receptors on a cell can produce different cellular responses even though they all signal through cAMP. While this conceptual framework has been an attractive idea, it has been difficult to explore experimentally. Recent work on cAMP diffusion shows that these signaling domains may be 10-100 nM in size, far below the resolution of light microscopy (Bock et al.,2020). This means that specialized signaling can be imaged in cellular appendages like the sensory cilium (Moore et al., 2016; Wu et al., 2021), but one cannot resolve the different signalsomes in the cell body because they are simply too small and too close together. How then can we begin to dissect the different signalsomes at the molecular level? Cattani-Cavalieri and colleagues took a novel approach to this problem, using differentiated human smooth airway cells. They expressed additional adenylyl cyclase 2 or 6 in these cells, and first demonstrated with the cADDis cAMP biosensor that these additional adenylyl cyclase pools produced extra normal levels of cAMP when stimulated with forskolin. The signalosome hypothesis would predict that these different enzymes, producing cAMP in different signalsomes, should produce different effects. The null hypothesis would be that the effects would be the same.
Cattani-Cavalieri and colleagues then stimulated the cells overexpressing either the adenylyl cyclase 2 or 6, and used mass spectrometry to determine which target proteins were phosphorylated. The results were beautiful new insights into the functional consequences of cAMP signalsomes. A few proteins were phosphorylated in both experimental conditions, but many were unique to the activation of one enzyme or the other. A molecular pattern is beginning to emerge in which receptor activation of different adenylyl cyclases, in different signalsomes, must activate different PKA enzymes, presumably anchored by different AKAPs, that in turn phosphorylate very different suites of target proteins. This fruitful experimental approach is only the beginning since there are other adenylyl cyclase isoforms, and one can imagine different pathways existing in different cell types. However the importance of this new experimental traction on a very old problem is quite exciting.
References
Bock, A., Annibale, P., Konrad, C., Hannawacker, A., Anton, S. E., Maiellaro, I., Zabel, U., Sivaramakrishnan, S., Falcke, M., & Lohse, M. J. (2020). Optical Mapping of cAMP Signaling at the Nanometer Scale. Cell, 182(6), 1519–1530.e17. https://doi.org/10.1016/j.cell.2020.07.035
Cattani-Cavalieri, I., Li, Y., Margolis, J., Bogard, A., Roosan, M. R., & Ostrom, R. S. (2023). Quantitative phosphoproteomic analysis reveals unique cAMP signaling pools emanating from AC2 and AC6 in human airway smooth muscle cells. Frontiers in Physiology, 14, 1149063. https://doi.org/10.3389/fphys.2023.1149063
Moore, B. S., Stepanchick, A. N., Tewson, P. H., Hartle, C. M., Zhang, J., Quinn, A. M., Hughes, T. E., & Mirshahi, T. (2016). Cilia have high cAMP levels that are inhibited by Sonic Hedgehog-regulated calcium dynamics. Proceedings of the National Academy of Sciences of the United States of America, 113(46), 13069–13074. https://doi.org/10.1073/pnas.1602393113
Wu, C.-T., Hilgendorf, K. I., Bevacqua, R. J., Hang, Y., Demeter, J., Kim, S. K., & Jackson, P. K. (2021). Discovery of ciliary G protein-coupled receptors regulating pancreatic islet insulin and glucagon secretion. Genes & Development, 35(17-18),1243–1255. https://doi.org/10.1101/gad.348261.121