Neurodegeneration Tools
Express bright fluorescent biosensors for cell stress and signaling with your neurodegenerative-associated protein of interest in your favorite neuronal culture or cell line. Examine ER stress, the UPR, and signaling biology in cells directly affected by neurodegenerative disease. Identify and optimize therapeutic compounds and express mutant proteins related to neurodegeneration.
Detect Disrupted Signaling in Neurodegeneration
Monitor changes in signaling biology from mutant protein expression and cell-based models of disease. Screen for compounds that restore normal cellular signaling. Examine disrupted cellular signaling associated with protein misfolding and ER stress.
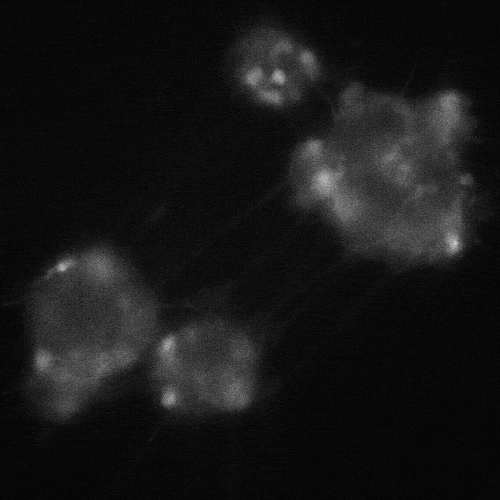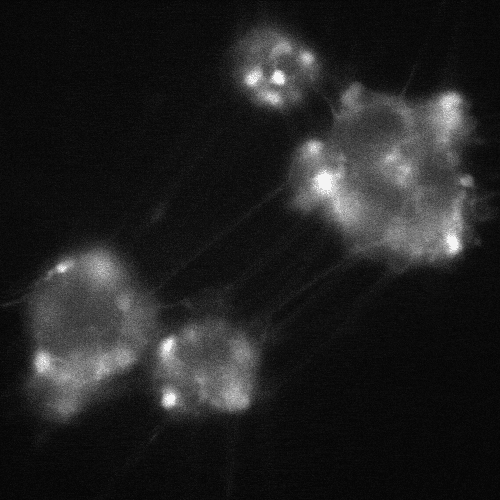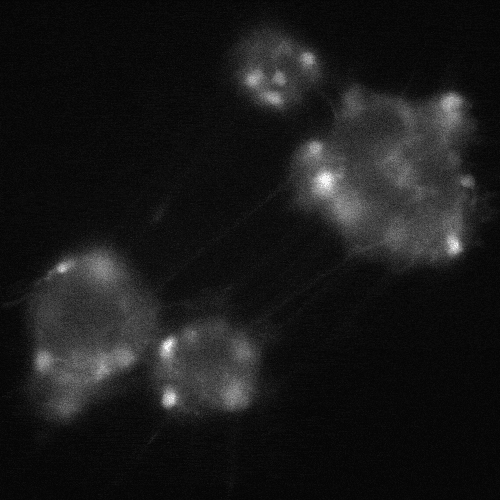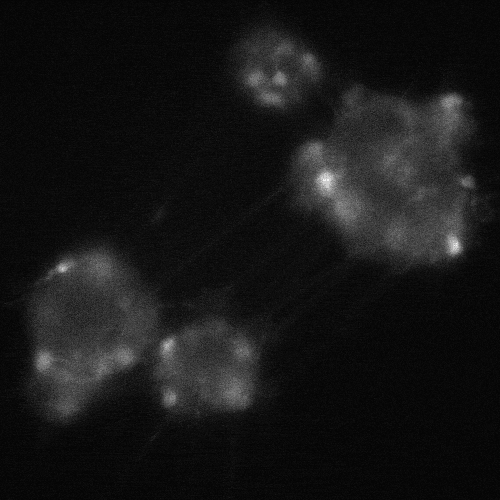
cADDis cAMP Sensor expression in BrainXell’s iPSC-derived motor neurons from patients with ALS. The neurons contain the D90A mutation in SOD1
Cell Stress
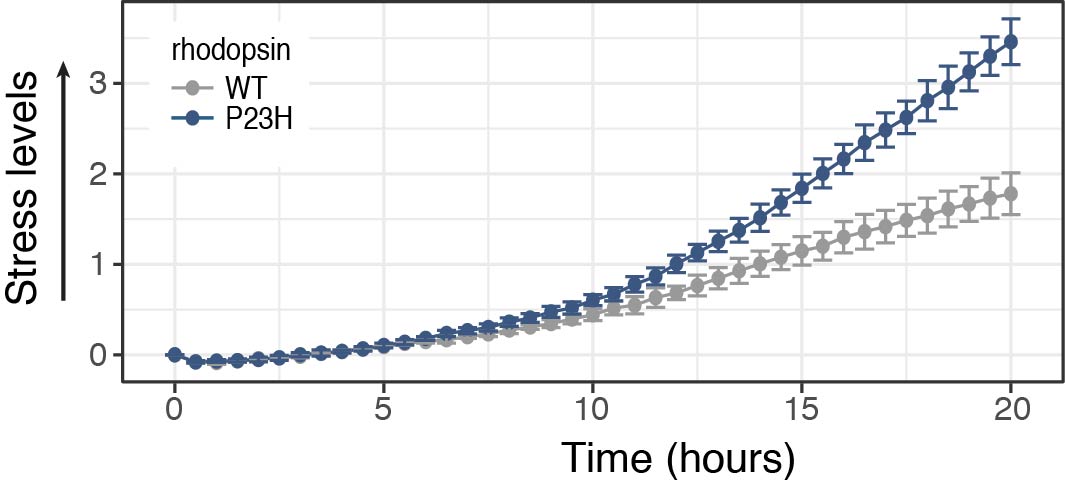
Protein misfolding and ER stress are implicated in many neurodegenerative diseases, including ALS and Retinitis Pigmentosa.
Detect ER stress from mutant proteins associated with neurodegeneration using our cell stress assays. Monitor responses over time and control for changes in protein expression with the Two-Color Cell Stress Assay.
Screen for compounds that inhibit or alleviate the stress response. The Two-Color assay is also a reversible indicator and can detect the onset and reduction of the stress response.
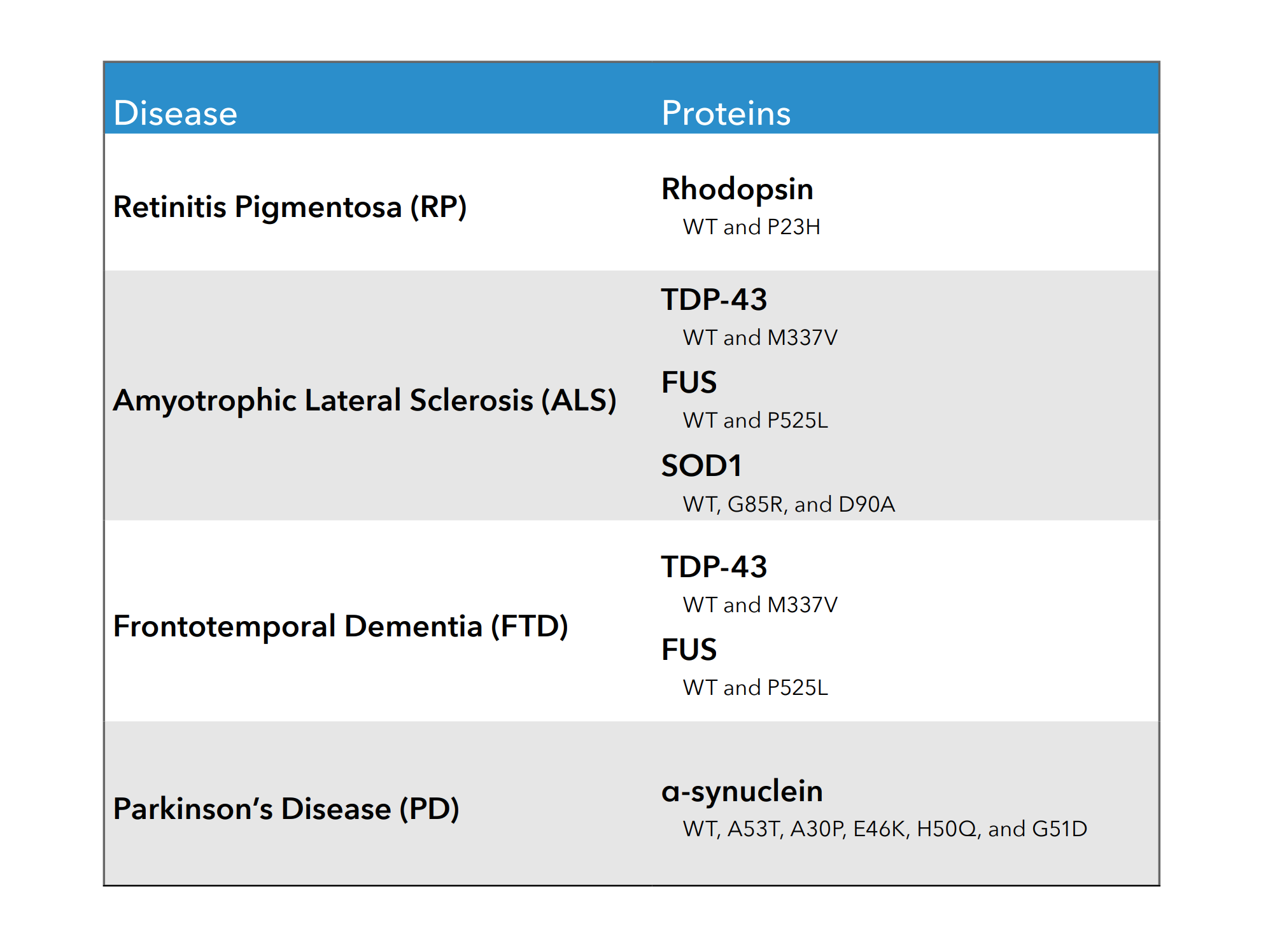
Neurodegenerative Proteins
Wild type and mutant proteins associated with neurodegenerative disease are available off-the-shelf in our BacMam vector. BacMam is an effective vector for expressing genes in primary and iPSC neurons, enabling detection in cell types relevant to neurodegeneration. BacMam allows for tightly controlled expression of these proteins, meaning they can be highly overexpressed or titrated to endogenous levels of expression. See below for example publications using Montana Molecular tools in neuronal cell types.
Are there other proteins you are interested in studying? Send an email to info@montanamolecular.com.
Neuronal Cell References
Primary Cultures, iPSCs, and in vivo assays
Area Postrema Neurons
- C. Zhang, et al. A brainstem circuit for nausea suppression. Cell Reports. June 2022.
- W. Han, et al. Nausea and the Brain: The Chemoreceptor Trigger Zone Enters the Molecular Age. Neuron. February 2021.
- C. Zhang, et al. Area Postrema Cell Types That Mediate Nausea-Associated Behaviors. Neuron. November 2020.
Astrocytes (Mouse)
- Z. Zhou, et al. Astrocytic cAMP modulates memory via synaptic plasticity. PNAS. January 2021.
Chromaffin Cells (Mouse)
- X. Chen, et al. Roles for PKC signaling in chromaffin cell exocytosis. Biophysical Journal. December 2024.
- X. Chen, et al. A PACAP-activated network for secretion requires coordination of Ca2+ influx and Ca2+ mobilization. Molecular Biology of the Cell. May 2024. (bioRxiv)
Cortical Neurons (Rat)
- E. Billard, et al. Pharmacological characterization of cannabidiol as a negative allosteric modulator of the 5-HT2A receptor. Cellular Signaling. January 2025.
Crz Neurons (Drosophilia)
- S. Thornquist, et al. Biochemical evidence accumulates across neurons to drive a network-level eruption. Molecular Cell. February 2021.
Dorsal Root Ganglia Neurons (Mouse, DRGs)
- J. Chadwick, et al. Dietary-dependent sensitization of neuronal leptin signaling promotes neural repair after injury via cAMP and gene transcription. Neuron. August 2025.
- L.Liu, et al. Gαq sensitizes TRPM8 to inhibition by PI(4,5)P2 depletion upon receptor activation. J.Neurosci. May 2019.
Hippocampal Neurons (Mouse)
- Paul G. DeCaen & Louise F. Kimura. Methods to Assess Neuronal Primary Cilia Electrochemical Signaling. Journal of Cellular Physiology. April 2025.
Hypothalamic Neurons (Mouse)
- I. Davies, et al. Chronic GIPR agonism results in pancreatic islet GIPR functional desensitisation. Molecular Metabolism. January 2025.
in vivo and tissue slice neurons (AAV delivery)
- C. Gao, et al. Semaglutide drives weight loss through cAMP-dependent mechanisms in GLP1R-expressing hindbrain neurons. bioRxiv. August 2025.
- J. Enriquez-Traba, et al. Dissociable control of motivation and reinforcement by distinct ventral striatal dopamine receptors. Nature Neuroscience. December 2024.
- S. Zhang, et al. Stochastic neuropeptide signals compete to calibrate the rate of satiation. Nature. November 2024. (bioRxiv)
- J. Alvarado et al. Transient cAMP production drives rapid and sustained spiking in brainstem parabrachial neurons to suppress feeding. Neuron. February 2024. (bioRxiv)
- A. Lutas et al. History-dependent dopamine release increases cAMP levels in most basal amygdala glutamatergic neurons to control learning. Cell Reports. January 2022.
- A. Lutas, et al. Dopamine-dependent cAMP dynamics in basal amygdala glutamatergic neurons. bioRxiv. September 2021.
- S. Zhang, et al. Hypothalamic dopamine neurons motivate mating through persistent cAMP signalling. Nature. August 2021.
Induced neural stem cells (Astrocytes, patient derived)
- J. Yu et al. N-3 polyunsaturated fatty acids promote astrocyte differentiation and neurotrophin production independent of cAMP in patient-derived neural stem cells. Mol. Psychiatry. June 2020.
iPSC-derived Neurons
- M. Ho, et al. Molecular mechanisms involved in alcohol craving, IRF3, and endoplasmic reticulum stress: a multi-omics study. Nature Translational Psychiatry. March 2024.
- K.M. Semesta, et al. The psychosis risk factor RBM12 encodes a novel repressor of GPCR/cAMP signal transduction. Journal of Biological Chemistry. August 2023.
- K. Harlen, et al. Live-cell biosensors reveal novel insight into neurotoxic and cardiotoxic compound mediated cellular stress response. Journal of Pharmacological and Toxicological Methods. October 2019.
Medium Spiny Neurons
- L. Ripoll, et al. Spatial organization of adenylyl cyclase and its impact on dopamine signaling in neurons. Nature Communications. September 2024.
Microglia
- E. Maguire, et al. The Alzheimer’s disease protective P522R variant of PLCG2, consistently enhances stimulus-dependent PLCγ2 activation, depleting substrate and altering cell function. bioRxiv. April 2020.
Oligodendrocyte Progenotior Cells (Human)
- C. Metrick, et al. GPR17 structure and agonism with small molecules and oxysterols. bioRxiv. November 2025.
Schwann Cells (Human)
- R. Nassini, et al. Targeting prostaglandin E2 receptor 2 in Schwann cells inhibits inflammatory pain but not inflammation. Nature Communications. September 2025.
- C. Sturaro, et al. Activation of peripheral NOP receptors reduces periorbital mechanical allodynia evoked by CGRP in mice. British Journal of Pharmacology. August 2025.
- R. Nassini, et al. Targeting the Schwann Cell EP2/cAMP Nanodomain to Block Pain but not Inflammation. bioRxiv. September 2024.
- F. De Logu, et al. Schwann cell endosome CGRP signals elicit peri orbital mechanical allodynia in mice. Nature Communications. February 2022.
- F. De Logu, et al. CGRP Signals from Endosomes of Schwann Cells to Elicit Migraine Pain. Research Square. August 2021.
Striatal Neurons
- L. Ripoll & M. von Zastrow. Spatial organization of adenylyl cyclase and its impact on dopamine signaling in neurons. bioRxiv. December 2023.
- M. Nuriya, et al. Alkyne-Tagged Dopamines as Versatile Analogue Probes for Dopaminergic System Analysis. Analytical Chemistry. July 2021.
- T. Patriarchi, et al. An expanded palette of dopamine sensors for multiplex imaging in vivo. Nature Methods. September 2020.
Trigeminal Ganglion Neurons
- Y. Kunioku, et al. Intracellular cAMP Signaling Pathway via Gs Protein-Coupled Receptor Activation in Rat Primary Cultured Trigeminal Ganglion Cells. Biomedicines. August 2023.
- N. Saito, et al. Gαs-Coupled CGRP Receptor Signaling Axis from the Trigeminal Ganglion Neuron to Odontoblast Negatively Regulates Dentin Mineralization. biomolecules. November 2022.
Cell Lines
Astrocytoma (Human, 1321N1)
- R. Matt, et al. Fingerprinting heterocellular β-adrenoceptor functional expression in the brain using agonist activity profiles. Frontiers in Molecular Biosciences. August 2023.
C6 Glioma
- N. Senese, et al. Antidepressants produce persistent Gαs associated signaling changes in lipid rafts following drug withdrawal. Molecular Pharmacology. May 2021.
- N. H. Wray, et al. NMDAR-independent, cAMP-dependent antidepressant actions of ketamine. Molecular Psychiatry. April 2, 2018.
- Singh et al. Disruption of lipid-raft localized Gαs/tubulin complexes by antidepressants: a unique feature of HDAC6 inhibitors, SSRI and tricyclic compounds. Neuropsychopharmacology. February 2018.
EOC20 (Microglial)
- King et al. A G protein‐coupled α7 nicotinic receptor regulates signaling and TNF‐α release in microglia. FEBS. July 2017.
GT1-7 (Immortalized Hypothalamic Neurons)
- H. Haddad, et al. Hypothalamic opsin 3 suppresses MC4R signaling and potentiates Kir7.1 to promote food consumption. PNAS. February 2025.
SH-SY5Y
- C. Byrnes, et al. 1-Deoxysphingolipids Require Very-Long-Chain Ceramide Synthesis to Induce ER Stress and Neurotoxicity. bioRxiv. December 2025.
- D. Wang, et al. Azoramide attenuates thapsigargin-induced ER stress and cell death in human SH-SY5Y cells via maintaining intracellular calcium homeostasis. European Journal of Pharmacology. November 2025.
- A. Ippolito, et al. Evidence that 5-HT2A receptor signalling efficacy and not biased agonism differentiates serotonergic psychedelic from non-psychedelic drugs. British Journal of Pharmacology. June 2025.
- A. Klein, et al. Inhibition of adenylyl cyclase 1 (AC1) and exchange protein directly activated by cAMP (EPAC) restores ATP-sensitive potassium (KATP) channel activity after chronic opioid exposure. bioRxiv. February 2025.
- I. Gkekas, et al. AI Promoted Virtual Screening, Structure-Based Hit Optimization, and Synthesis of Novel COVID-19 S-RBD Domain Inhibitors. Journal of Chemical Information and Modeling. November 2024.
- A. Ippolito, et al. Increased 5-HT2A receptor signalling efficacy differentiates serotonergic psychedelics from non-psychedelics. bioRxiv. June 2024.
- K. Harlen, et al. Live-Cell Assays for Cell Stress Responses Reveal New Patterns of Cell Signaling Caused by Mutations in Rhodopsin, α-Synuclein and TDP-43 Front. Cell.
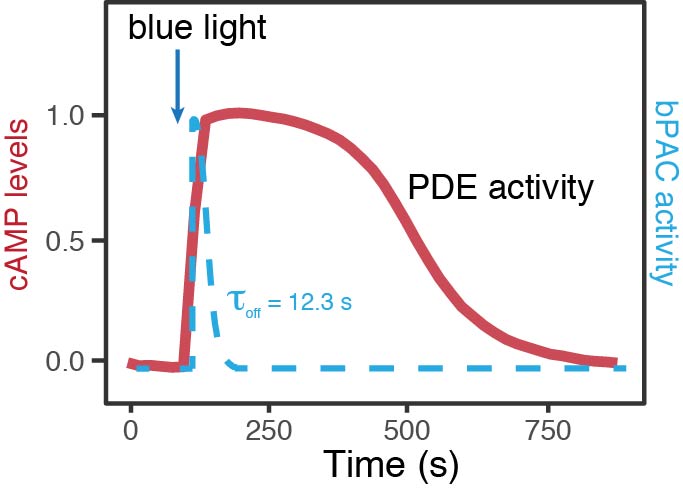
Optogenetics: Light-Activated Adenylyl Cyclase
Express wild type and mutant proteins in your cells of interest, then use a bacterial Photoactivated Adenylyl Cyclase (bPAC) to manipulate intracellular cAMP. Monitor intracellular cAMP levels and characterize changes in PDE activity using the red cADDis cAMP sensor. Request a Manuscript to see further experimental details, or see the Optogenetics page.
Contact Us
If you have any questions about using these tools in your cells or would like to request a quote, send us an email at info@montanamolecular.com. We’re happy to help!