Find your next-generation SARS-CoV-2 inhibitor using mutant 3CL proteases
The SARS-CoV-2 protease is crucial to virus replication. If the main protease, known as 3CLpro can be blocked, the virus can be stopped. The commonly prescribed Paxlovid contains a 3CLpro inhibitor known as nirmatrelvir, which is an active antiviral compound. Unfortunately, there is a growing body of evidence that a few mutations in 3CLpro can both block nirmatrelvir’s effect and preserve enzymatic activity.
Several mutations have arisen naturally and have been identified in patients. (Shu and McCauley 2017; Hu et al. 2023; Huang et al. 2023). Thankfully, these mutations do not yet occur in widely distributed variants of concern, but a new generation of inhibitors will soon be necessary if they become more prevalent. Other, very similar mutations arose in laboratory settings where the SARS-CoV-2 virus was passaged under nirmatrelvir selection. (Iketani et al. 2023; Pfizer Laboratories 2022). Several of these caused more than 10-fold changes in resistance to nirmatrelvir.
Shift in nirmatrelvir IC50 seen in mutant 3CL proteases C1200N-C1205N
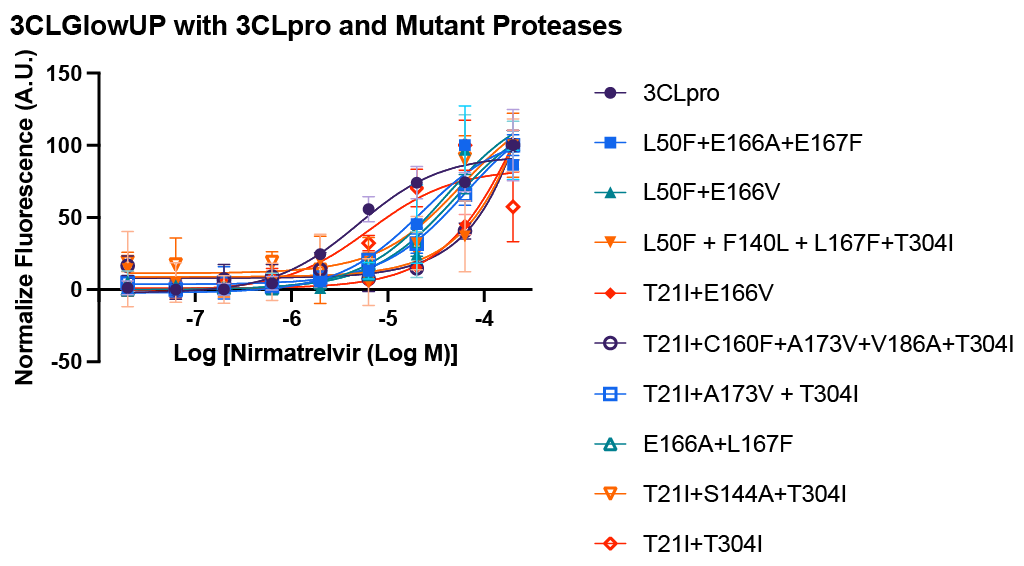
As SARS-CoV-2 continues to evolve, successful inhibition of these mutated proteases becomes increasingly important. We built a suite of mutant 3CLpro ‘s that have been found to shift Nirmatrelvir’s IC50, and paneled these against our 3CLGlowUp biosensor – demonstrating shifted IC50 curves.
Shift in nirmatrelvir IC50 seen in mutant 3CL proteases C1206N-C1215N
Use these tools on your search for new 3CLpro inhibitors to screen the activity of your compounds against a landscape of 3CLpro mutations. Please reach out if you would like Montana Molecular to test your compounds for you. We offer a variety of assay services and would be happy to discuss your project!
The following mutants are available and have demonstrated strong nirmatrelvir resistance with the following substitutions:
T21I (C1200N) – T21I is commonly found in the B.1.1.318 variant (Ullrich et al. 2022). Structurally, this mutation resides in the S4′ subsite of the 3CLpro enzyme, and it has been shown to have higher catalytic activity compared to the wild-type 3CLpro in kinetic assays (Duan et al. 2023). Ullrich et al. reported that T21I remained susceptible to nirmatrelvir when using a biochemical FRET assay (Ullrich et al. 2022). However, in a recombinant SARS-CoV-2 assay in huh7-ACE2 cells, T21I exhibits a 4.6-fold IC50 resistance to nirmatrelvir (Iketani et al. 2023). This mutation has been identified in patients and reported to the GISAID database (Shu and McCauley 2017).
L50F (C1201N) – L50F is a compensatory substitution that aids in maintaining the virus’s fitness (Jochmans et al. 2023; Zhou et al. 2022; Iketani et al. 2023). In a recombinant SARS-CoV-2 assay conducted in huh7-ACE2 cells, L50F has a 4.2-fold resistance to nirmatrelvir (Iketani et al. 2023). Structurally, the L50F mutation is located in the S2 subsite of 3CLpro and demonstrates increased catalytic activity compared to wild-type 3CLpro in kinetic assays (Duan et al. 2023). This mutation has been detected in patients and documented in the GISAID database (Shu and McCauley 2017).
E166V (C1202N) – In a recombinant SARS COV-2 assay conducted in huh7-ACE2 cells, the E166V mutation demonstrates a 100-fold IC50 resistance to nirmatrelvir, albeit with a reduction in fitness (Iketani et al. 2023). This mutation has been detected in patients and reported to the GISAID database (Shu and McCauley 2017; Iketani et al. 2023). Interestingly, the prevalence of E166V is higher in subjects treated with nirmatrelvir compared to those in the control group (0.8% versus less than 0.2%, respectively) (Pfizer Laboratories 2022).
E166G (C1203N) – The E166G mutation seems to be associated with reduced enzymatic activity and increased Ki values for nirmatrelvir, according to Hu et al. and Tan et al. (Tan et al. 2023; Hu et al. 2023) In both studies, E166G exhibited a 7.4-fold reduction in enzymatic activity, and a 16.4-fold increase in the Ki value for nirmatrelvir (Tan et al. 2023; Hu et al. 2023). Additionally, this mutation has been detected in patients and documented in the GISAID database (Shu and McCauley 2017).
Q189E (C1204N) – Q189E, located within 6 Å of the nirmatrelvir binding site, is a naturally occurring mutation and has a two-fold increase in enzymatic activity compared to wild type 3CLpro (Hu et al. 2023). In a biochemical assay, Q189E reported a 19-fold increase in IC50 to nirmatrelvir resistance (Sasi et al. 2022). Additionally, a FRET-based enzymatic assay revealed a 10.7-fold increase in IC50 of nirmatrelvir (Huang et al. 2023).
H172Y (C1205N) – H172Y substitution results in reduced stability, and enzyme activity, with the IC50 of nirmatrelvir against the H172Y mutant being 24-fold greater, as measured by an enzymatic assay (de Oliveira et al. 2022). Huang et al. reported a 30.7-fold increase in the IC50 of nirmatrelvir using a FRET-based enzymatic assay (Huang et al. 2023). Additionally, the Ki increased by 146-fold, as measured by an enzymatic assay (Hu et al. 2023). In a biochemical assay using recombinant SARS-CoV-2 3CLpro containing amino acid substitution H172Y, Pfizer had reported a 250 fold increase in Ki (Pfizer Laboratories 2022).
E166A + L167F (C1206N) – In VeroE6 cells, employing a FRET-based assay with a presence of .5µM CP 100356, the E166A, L167F mutation led to a 10-fold change in the EC50 (Jochmans et al. 2023). This mutation is a component of the in vitro emergent mutation L50F/E166A/L167F (Jochmans et al. 2023).
T21I + T304I (C1207N) – The T21I T304I variant emerged during escape studies under nirmatrelvir inhibition (Zhou et al. 2022). Using a short-term concentration response treatment in VeroE6 cells against the escape mutant T21I T304I, researchers observed a 5.9-fold shift in EC50 resistance to nirmatrelvir (Zhou et al. 2022). Pfizer reported a 3 to 8-fold increase in EC50 to nirmatrelvir using a biochemical assay (Pfizer Laboratories 2022).
T21I + E166V (C1208N) – In a recombinant SARS-COV-2 assay conducted in huh7-ACE2 cells, the E166V mutation alone displayed a 100-fold IC50 resistance to nirmatrelvir, although it led to reduced fitness (Iketani et al. 2023). However, when combined with the T21I mutation, virus replication was restored (Iketani et al. 2023) resulting in an 83-fold change in nirmatrelvir resistance (Iketani et al. 2023). This T21I, E166V mutation has been identified in patients and reported to the GISAID database (Shu and McCauley 2017; Iketani et al. 2023). Pfizer also reported an 83-fold increase in EC50 to nirmatrelvir using a biochemical assay (Pfizer Laboratories 2022).
L50F + E166V (C1209N) – In a recombinant SARS-COV-2 assay conducted in huh7-ACE2 cells, the E166V mutation alone displayed a 100-fold IC50 resistance to nirmatrelvir, although it led to reduced fitness (Iketani et al. 2023). However, when combined with the mutation L50F, virus replication was restored, resulting in a 53-fold increase in IC50 to nirmatrelvir (Iketani et al. 2023). The L50F, E166V mutation has been detected in patients and reported to the GISAID database (Shu and McCauley 2017; Iketani et al. 2023). Pfizer also reported a 34 to 175-fold increase in EC50 to nirmatrelvir using a biochemical assay (Pfizer Laboratories 2022).
L50F + E166A + L167F (C1210N) – In biochemical analysis, nirmatrelvir’s IC50 value was found to be 72-fold greater in the presence of the L50F, E166A, L167F mutation (Jochmans et al. 2023). In a HEK293T cell-based 3CLpro fluorescent reporter assay nirmtrelvir EC50 increased from .96µM to 27µM with this mutation (Jochmans et al. 2023). These mutations occur independently in patients and have emerged collectively when passing the virus under nirmatrelvir selection (Jochmans et al. 2023; Shu and McCauley 2017). Furthermore, a FRET-based enzymatic assay demonstrated a 51.9-fold increase in IC50 values for nirmatrelvir in the presence of this mutation (Huang et al. 2023).
T21I + S144A + T304I (C1211N) – Tong et al. reported a 10-fold increase in IC50 for GC376 using a FRET-based endpoint assay (Tong et al. 2023). Pfizer reported a 27.8-fold increase in EC50 to nirmatrelvir using a biochemical assay (Pfizer Laboratories 2022).
T21I + A173V + T304I (C1212N) – Pfizer reported a 15-fold increase in EC50 to nirmatrelvir using a biochemical assay with the T21I, A173V, T304I mutation (Pfizer Laboratories 2022).
T21I + L50F + A193P + S301P (C1213N) – Tong et al. reported a 3.6-fold increase in IC50 for GC376 using a FRET-based endpoint assay with this mutation (Tong et al. 2023). Additionally, Pfizer reported a 28.8-fold increase in EC50 to nirmatrelvir using a biochemical assay (Pfizer Laboratories 2022).
L50F + F140L + L167F + T304I (C1214N) – Pfizer reported a 54.7-fold increase in EC50 to nirmatrelvir using a biochemical assay with this mutation (Pfizer Laboratories 2022).
T21I + C160F + A173V + V186A + T304I (C1215N) – Tong et al. reported a 4.9-fold increase in IC50 for GC376 using a FRET-based endpoint assay with this mutation (Tong et al. 2023). Pfizer reported a 28.5-fold increase in EC50 to nirmatrelvir using a biochemical assay (Pfizer Laboratories 2022).
Are there other 3CLpro mutations you are interested in testing? Do you have questions regarding these tools? We’d love to hear from you!
References
de Oliveira, V., Ibrahim, M., Sun, X., Hilgenfeld, R., & Shen, J. (2022). H172Y Mutation Perturbs the S1 Pocket and Nirmatrelvir Binding of SARS-COV-2 Main Protease through a Nonnative Hydrogen Bond. https://doi.org/10.21203/rs.3.rs-1915291/v1
Duan, Y., Zhou, H., Liu, X., Iketani, S., Lin, M., Zhang, X., Bian, Q., Wang, H., Sun, H., Hong, S. J., Culbertson, B., Mohri, H., Luck, M. I., Zhu, Y., Liu, X., Lu, Y., Yang, X., Yang, K., Sabo, Y., … Yang, H. (2023). Molecular mechanisms of SARS-COV-2 resistance to Nirmatrelvir. Nature, 622(7982), 376–382. https://doi.org/10.1038/s41586-023-06609-0
Hu, Y., Lewandowski, E. M., Tan, H., Zhang, X., Morgan, R. T., Zhang, X., Jacobs, L. M., Butler, S. G., Gongora, M. V., Choy, J., Deng, X., Chen, Y., & Wang, J. (2023). Naturally occurring mutations of SARS-COV-2 main protease confer drug resistance to Nirmatrelvir. ACS Central Science, 9(8), 1658–1669. https://doi.org/10.1021/acscentsci.3c00538
Huang, C., Shuai, H., Qiao, J., Hou, Y., Zeng, R., Xia, A., Xie, L., Fang, Z., Li, Y., Yoon, C., Huang, Q., Hu, B., You, J., Quan, B., Zhao, X., Guo, N., Zhang, S., Ma, R., Zhang, J., … Yang, S. (2023). A new generation mpro inhibitor with potent activity against SARS-COV-2 omicron variants. Signal Transduction and Targeted Therapy, 8(1). https://doi.org/10.1038/s41392-023-01392-w
Iketani, S., Mohri, H., Culbertson, B., Hong, S. J., Duan, Y., Luck, M. I., Annavajhala, M. K., Guo, Y., Sheng, Z., Uhlemann, A.-C., Goff, S. P., Sabo, Y., Yang, H., Chavez, A., & Ho, D. D. (2023). Multiple pathways for SARS-COV-2 resistance to Nirmatrelvir. Nature, 613(7944), 558–564. https://doi.org/10.1038/s41586-022-05514-2
Jochmans, D., Liu, C., Donckers, K., Stoycheva, A., Boland, S., Stevens, S. K., De Vita, C., Vanmechelen, B., Maes, P., Trüeb, B., Ebert, N., Thiel, V., De Jonghe, S., Vangeel, L., Bardiot, D., Jekle, A., Blatt, L. M., Beigelman, L., Symons, J. A., … Vandyck, K. (2023). The substitutions L50F, E166A, and L167F in SARS-COV-2 3clpro are selected by a protease inhibitor in vitro and confer resistance to Nirmatrelvir. mBio, 14(1). https://doi.org/10.1128/mbio.02815-22
Pfizer Laboratories. (2022). (rep.). FACT SHEET FOR HEALTHCARE PROVIDERS: EMERGENCY USE AUTHORIZATION FOR PAXLOVID. Retrieved from https://labeling.pfizer.com/ShowLabeling.aspx?id=16474.
Sasi, V. M., Ullrich, S., Ton, J., Fry, S. E., Johansen-Leete, J., Payne, R. J., Nitsche, C., & Jackson, C. J. (2022). Predicting antiviral resistance mutations in SARS-COV-2 main protease with computational and experimental screening. Biochemistry, 61(22), 2495–2505. https://doi.org/10.1021/acs.biochem.2c00489
Shu, Y., & McCauley, J. (2017). GISAID: Global initiative on sharing all influenza data – from vision to reality. Eurosurveillance, 22(13). https://doi.org/10.2807/1560-7917.es.2017.22.13.30494
Tan, B., Joyce, R., Tan, H., Hu, Y., & Wang, J. (2023). SARS-COV-2 main protease drug design, Assay Development, and Drug Resistance Studies. Accounts of Chemical Research, 56(2), 157–168. https://doi.org/10.1021/acs.accounts.2c00735
Tong, X., Keung, W., Arnold, L. D., Stevens, L. J., Pruijssers, A. J., Kook, S., Lopatin, U., Denison, M., & Kwong, A. D. (2023). Evaluation of in vitro antiviral activity of SARS-COV-2 Mpro inhibitor pomotrelvir and cross-resistance to nirmatrelvir resistance substitutions. Antimicrobial Agents and Chemotherapy, 67(11). https://doi.org/10.1128/aac.00840-23
Ullrich, S., Ekanayake, K. B., Otting, G., & Nitsche, C. (2022). Main protease mutants of SARS-COV-2 variants remain susceptible to Nirmatrelvir. Bioorganic & Medicinal Chemistry Letters, 62, 128629. https://doi.org/10.1016/j.bmcl.2022.128629
Zhou, Y., Gammeltoft, K. A., Ryberg, L. A., Pham, L. V., Tjørnelund, H. D., Binderup, A., Duarte Hernandez, C. R., Fernandez-Antunez, C., Offersgaard, A., Fahnøe, U., Peters, G. H., Ramirez, S., Bukh, J., & Gottwein, J. M. (2022). Nirmatrelvir-resistant SARS-COV-2 variants with high fitness in an infectious cell culture system. Science Advances, 8(51). https://doi.org/10.1126/sciadv.add7197